Abstract
Childhood trauma (CT) is a well‐established risk factor for major depressive disorder (MDD). However, the underlying mechanism linking CT and MDD remains not fully understood. The present study tested the hypothesis that CT have effects on specific types of anhedonia in depression via reward system. To do so, we evaluated different aspects of anhedonia and resting‐state functional connectivity (FC) in reward system among 66 patients with MDD (44 with moderate‐to‐severe and 22 with no or low CT), and 57 healthy controls (HC; 23 with moderate‐to‐severe and 34 with no or low CT). Results showed that MDD patients with moderate‐to‐severe CT suffered more severe state anhedonic depression than patients with no or low level of CT. Individuals with moderate‐to‐severe CT, irrespective of MDD diagnosis, had elevated physical, social and anticipatory but not consummatory trait anhedonia, and demonstrated decreased left nucleus accumbens (NAcc)‐right orbital frontal cortex (OFC) and left ventral caudate‐left OFC connectivity compared to those with no or low exposure. Left NAcc‐right OFC connectivity mediated relationship between CT and state anhedonia in MDD. The total altered ventral striatum (VS)‐OFC connectivity mediated links between CT and physical trait anhedonia in HC. These findings highlight specific types of anhedonia and the core reward system as targets of CT. Blunted hedonic responses via decreased coupling within core reward system may be involved in the mechanism of depression following CT. Implications for clinical interventions are also discussed.
Keywords: Anhedonia, childhood trauma, depression, resting‐state functional connectivity, reward circuit
1. INTRODUCTION
Major depressive disorder (MDD) is a severe mental disorder with a heterogeneous clinical syndrome. Previous studies have revealed that childhood trauma (CT) is a potent risk factor for the development of MDD; however, which depressive symptomatology is affected by CT and the involving mechanism(s) are inadequately understood.
Anhedonia, a core symptom of depression, defined as the inability to experience pleasure, can vary independently of other depressive symptomatology (Drysdale et al., 2017; Molet et al., 2016). A prior animal study from our lab found that different stressors may induce different depression phenotypes, and specifically, early maternal deprivation was associated with more severe anhedonia (Bai et al., 2014). Induction of anhedonia‐like depression behaviors arising from early‐life maltreatment has also been reported in other animal studies (Bolton et al., 2018; Molet et al., 2016). Several human studies also revealed the association between CT and elevated anhedonia (Agrawal et al., 2012; Dillon et al., 2009; Germine, Dunn, McLaughlin, & Smoller, 2015). These studies suggest that when taking the symptom dimension into consideration, the CT may mainly have effects on anhedonia aspect of MDD.
However, it should be noted that anhedonia is not a unitary concept as it can be parsed into different aspects according to different criteria. For example, according to the content of the pleasurable stimulus, there are physical and social components of anhedonia (Chapman, Chapman, & Raulin, 1976). Based on the different cognitive phase of reward process, anhedonia can be divided into anticipatory as well as consummatory aspects (Gard, Gard, Kring, & John, 2006). Moreover, anhedonia can be manifested as either a clinical state or an enduring personality trait (A. S. Cohen, Najolia, Brown, & Minor, 2011). The limited number of prior human studies only investigated the relationships between CT and few aspects of anhedonia, such as the relationship between CT and the state anhedonia (Agrawal et al., 2012; Dillon et al., 2009) as well as the social anhedonia (Germine et al., 2015). An important question remains to explore is whether CT affects all types of anhedonia or it is just associated with specific types of anhedonia.
Anhedonia is strongly associated with dysfunction in the brain's reward system (Stringaris et al., 2015), which referred to a frontal‐striatal circuit with the key structures including the striatum, the orbital prefrontal cortex (OFC) and the anterior cingulate cortex (ACC) (Haber & Knutson, 2010). Though other brain structures may be involved in the broad reward‐related processes, previous studies have revealed that the major players in the reward system, example. striatum, OFC and ACC responded to general reward processing and be responsible for distinct aspects of anhedonia (Haber & Knutson, 2010; Liu, Hairston, Schrier, & Fan, 2011). Previous studies have revealed that CT may have impacts on the development of the abovementioned core reward circuit (Boecker et al., 2014; Chaney et al., 2014; Hanson et al., 2016; Hanson, Hariri, & Williamson, 2015). These findings further lead to the hypothesis that CT may have effects on different aspects of anhedonia via the core reward circuitry. Besides, as amygdala plays a very important role in the onset and progress of depression, and studies also suggest that amygdala contributes to positive emotion and reward (Murray, 2007), the role of amygdala in the mechanism of anhedonia following CT was also included.
Several previous studies have explored the role of reward‐related alterations in the effects of CT on overall depression level while not on the depressive dimensional outcome of anhedonia (Casement et al., 2014; Hanson et al., 2015; Romens et al., 2015). There is robust heterogeneity in symptom presentation in MDD, involving a variety of neural bases and developmental trajectories (Drysdale et al., 2017; Pine, Cohen, Cohen, & Brook, 1999). Previous studies have revealed that low activity in the ventral striatum (VS) to rewards is specific to anhedonia but not to low mood or other depressive symptoms in MDD (Stringaris et al., 2015; Wacker, Dillon, & Pizzagalli, 2009). In light of these findings, further studies investigating the link between CT and reward‐related alterations on the specific symptom, anhedonia, instead of directly the depression episode or other depression symptoms are needed to clarify the pathogenesis of MDD following CT.
Resting‐state functional connectivity (FC), indexed by correlations in low‐frequency fluctuation of the resting‐state functional magnetic resonance imaging (fMRI) signal, is one of the commonly used methods to investigate the coordination and interaction of neural activity between anatomically distributed but functionally related brain regions (Fox & Raichle, 2007). By using this method, previous studies have detected that the FC within reward circuitry were altered in various psychiatric conditions (Costa Dias et al., 2015; Dandash et al., 2014; Gabbay et al., 2013; Lips et al., 2014; Satterthwaite et al., 2015; Wang et al., 2016; Wilcox, Teshiba, Merideth, Ling, & Mayer, 2011; Zhu et al., 2017) and these alterations could help predict treatment response (Downar et al., 2014), distinguish symptom (Gabbay et al., 2013) and characterize disease heterogeneity (Costa Dias et al., 2015). Reward system FC at rest were also found to be highly concordant with brain reward system activation during task, and both of them could predict the individual reward‐related behavioral performance (Dong, Li, Wang, & Potenza, 2018; Li et al., 2013; Satterthwaite et al., 2015). These studies suggest that though being task‐free, the resting‐state FC is a reliable method to evaluate the reward system functioning.
Taken together, the present study was designed to investigate whether CT has effects on specific aspects of anhedonia via the alterations in the reward‐system. To do so, patients with MDD and HC with different levels of CT were included. Different types of anhedonia were measured and resting‐state FC was assessed to evaluate reward circuit functioning. We first established the effects of CT on different components of anhedonia and the reward‐related FC. Then the mediation effects of FC alterations on the links between CT and anhedonia were examined. Our hypotheses were as follows: (a) CT is associated with severe anhedonia and decreased coupling within the reward system in both MDD patients and HC though the alterations in MDD might be more severe; (b) FC alterations within the reward system mediate the relationship between CT and anhedonia in both MDD patients and HC, suggesting the mechanisms linking CT to MDD.
2. METHODS AND MATERIALS
2.1. Subjects
A total of 66 first‐episode, drug‐naive MDD patients and 57 HC participated in the present study. MDD patients were recruited from the psychology clinic at the Second Xiangya Hospital of Central South University, Changsha, Hunan, China. All patients were experiencing a first episode of depression and had never received psychotropic medication. Exclusion criteria included any axis I psychiatric disorder comorbidity and a history of major medical or neurological problems. Two experienced psychiatrists confirmed the diagnosis of MDD and comorbidity for each patient according to the Structural Clinical Interview for the DSM‐IV Axis I (SCID‐I).
Subjects in the HC group were recruited from several colleges and communities in Changsha. Control subjects were also screened for psychiatric disorders by two experienced psychiatrists using the SCID‐I. Exclusion criteria were history of any psychiatric illnesses and any major medical or neurological problems.
All participants were right‐handed, 18–35 years old, and had at least 9 years of formal education. The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and all the subjects provided written informed consent.
2.2. Clinical assessments
2.2.1. Assessment of CT and group classification of with moderate‐to‐severe and with no or low CT
Maltreatment experiences during childhood were assessed by the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1994), which was comprised of five factors: emotional, physical and sexual abuse, and emotional and physical neglect. Cut‐off scores for moderate‐to‐severe CT as specified in the CTQ manual (Bernstein & Fink, 1998) were used to create dichotomous variables of exposure for each CTQ subscale (physical neglect ≥ 10, physical abuse ≥ 10, emotional neglect ≥ 15, emotional abuse ≥ 13, sexual abuse ≥ 8). Participants who reported having a moderate‐to‐severe CT on at least one subscale were classified into with moderate‐to‐severe CT group, and those reporting no or low exposure to all five types of CT were classified into with no or low CT group. This criteria for the CT dichotomous group classification has been used in many previous studies (Chaney et al., 2014; Dannlowski et al., 2012; Moog et al., 2018; Tyrka, Wyche, Kelly, Price, & Carpenter, 2009).
2.2.2. Assessments of different aspects of anhedonia
Physical and social anhedonia were measured by the 61‐item revised Physical Anhedonia Scale (PAS) (Chapman & Chapman, 1978) and the 40‐item Social Anhedonia Scale (SAS) (Eckblad, Chapman, Chapman, & Mishlove, 1982) respectively. The anticipatory and consummatory aspects of anhedonia were evaluated by the Temporal Experience of Pleasure Scale (TEPS) (Gard et al., 2006). TEPS consists of two subscales designed for the assessment of anticipatory (TEPS_ANT) and consummatory pleasure (TEPS_CON). PAS, SAS, and TEPS all require the participants to report how true the hedonic experience is for them in general and thus are all trait measures.
State anhedonia was measured by the total score of items on the Beck Depression Inventory (Beck, 1965) associated with anhedonia symptoms: item 4 (loss of pleasure), item 12 (loss of interest), and item 21 (loss of interest in sex). The use of these three items as an index of state anhedonia has been adopted in many previous studies and has demonstrated acceptable reliability (Ballard et al., 2017; Joiner, Brown, & Metalsky, 2003). In addition, the remaining 18 BDI items were totaled to form an assessment of nonanhedonic depressive symptoms.
2.2.3. Other assessments
The Edinburgh Handedness Inventory (EHI) (Oldfield, 1971), the State Trait Anxiety Inventory (Spielberger et al., 1983), the Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960) and the Perceived Stress Scale (PSS) (S. Cohen, Kamarck, & Mermelstein, 1983) were also administered to participants. The PSS, an assessment of perceived stress in the last month, has been previously used to control for the potential confounding effects caused by more recent stressors in studies on the impacts of childhood stress (Dannlowski et al., 2012; Grosse et al., 2016).
2.3. Imaging procedures
2.3.1. Image acquisition and preprocessing
Imaging data were acquired on a Siemens Skyra 3‐T magnetic resonance scanner at the Second Xiangya Hospital of Central South University. The resting‐state fMRI series were collected using the echoplanar imaging sequence with the parameters: 2500‐ms repetition time (TR), 25‐ms echo time (TE), 39 axial slices, 3.5‐mm slice thickness, no gap, 3.8 × 3.8 × 3.5‐mm voxel size, 200 volumes, 90° flip angle, 240‐mm field of view, and 64 × 64 data matrix. In addition, the three‐dimensional T1‐weighted, magnetization‐prepared rapid gradient echo (MPRAGE) sagittal images were acquired. The parameters were 1900‐ms TR, 2.01‐ms TE, 176 slices, 1.00‐mm slice thickness, 1.0 × 1.0 × 1.0‐mm voxel size, 9° flip angle, 900‐ms inversion time, 256‐mm field of view, and 256 × 256 matrix.
Data were preprocessed using the Data Processing Assistant for Resting‐State fMRI (DPARSF V2.3) (Yan & Zang, 2010). The following stepwise procedures were conducted: removal of the first 10 volumes; slice time correction; realignment of head motion; normalization to the Montreal Neurological Institute (MNI) atlas; spatial smoothing (full‐width at half maximum = 8 mm); linear detrending; regressing out of nuisance covariates (six head motion parameters, white matter signal, cerebrospinal fluid signal and global signal); and temporal band‐pass filtering (0.01 Hz—0.08 Hz). One subject (MDD with moderate‐to severe CT) did not perform the fMRI scan due to his schedule issue. Data from two subjects were excluded from further image analysis with one (MDD with moderate‐to‐severe CT) due to bad normalization and the other due to excessive head motion (MDD with no or low CT, translation or rotation exceeded ±1.5 mm or ±1.5°).
2.3.2. Head movement
The effects of head movement were assessed and managed based on the methods proposed by Power, Barnes, Snyder, Schlaggar, and Petersen (2012). First, the frame‐wise displacement (FD) from the translation and rotation parameters for each subject was calculated. Second, a “scrubbing routine” was used to censor any frame with an FD > 0.5 mm from the following seed‐based FC calculation. Third, as described below, the mean FD was further controlled as a covariate in the imaging statistical analysis. After scrubbing, all including subjects had an FD ≤0.16 mm and at least 76% of frames remained to be calculated, which satisfied the analyzable requirements (60%) outlined in Power et al. (2012) (see mean FD and %frames censored in Table 1).
TABLE 1.
Demographical and clinical characteristics among four groups
| Characteristics | MDD | HC | F/t/χ 2 | p | ||
|---|---|---|---|---|---|---|
| Moderate‐to‐severe CT (N = 44) | No or low CT (N = 22) | Moderate‐to‐severe CT (N = 23) | No or low CT (N = 34) | |||
| Age (years) | 23.86 (5.06) | 23.09 (4.83) | 19.70 (0.97) | 20.53 (2.21) | 8.39 | < .001 |
| Gender (female, %) | 25 (56.8) | 16 (72.7) | 13 (56.5) | 17 (50.0) | 4.30 | .231 |
| Education (years) | 14.23 (2.03) | 13.95 (2.61) | 13.04 (0.71) | 13.65 (0.30) | 2.05 | .110 |
| Duration (weeks) | 29.28 (22.15) | 20.36 (17.00) | — | — | 1.65 | .103 |
| Age onset | 22.42 (5.71) | 21.23 (6.96) | — | — | 0.66 | .515 |
| HAMD | 21.68 (6.55) | 20.50 (6.69) | 1.22 (1.68) | 1.03 (1.80) | 167.49 | <.001 |
| PSS | 27.64 (6.56) | 24.68 (6.83) | 16.78 (5.62) | 13.53 (4.67) | 42.43 | <.001 |
| CTQ_total | 54.82 (11.87) | 36.36 (5.07) | 48.13 (11.35) | 32.65 (4.55) | 43.95 | <.001 |
| Physical neglect | 11.61 (3.18) | 7.51 (1.43) | 11.70 (3.46) | 6.71 (1.40) | 32.86 | <.001 |
| Physical abuse | 7.89 (3.22) | 5.32 (0.65) | 7.00 (3.15) | 5.41 (0.96) | 9.06 | <.001 |
| Emotional neglect | 17.86 (3.83) | 10.18 (3.17) | 13.83 (4.89) | 8.79 (2.74) | 44.85 | <.001 |
| Emotional abuse | 11.16 (4.66) | 7.64 (2.56) | 8.96 (3.51) | 6.56 (1.56) | 12.72 | <.001 |
| Sexual abuse | 6.30 (2.86) | 5.82 (1.37) | 6.65 (2.12) | 5.18 (0.39) | 2.99 | <.05 |
| FD | 0.07 (0.28) | 0.08 (0.03) | 0.08 (0.02) | 0.08 (0.03) | 0.43 | .733 |
| Frames censored (%) | 0.04 (0.07) | 0.01 (0.02) | 0.02 (0.04) | 0.02 (0.04) | 1.82 | .147 |
Means with standard deviations in parentheses. F//t/χ 2: Variables of age, PSS, CTQ assessments, HAMD, FD and frames censored were tested by one‐way ANOVA as indicated by F; Categorical data was tested using chi‐square test as indicated by χ 2; variables such as age onset and illness duration were tested by two‐sample t‐test as indicated by t. Significant post hoc tests (p < .05, Bonferroni corrected): age: MDD_moderate‐to‐severe CT > HC_moderate‐to‐severe CT = HC_no or low CT; MDD_no or low CT > HC_moderate‐to‐severe CT; PSS: MDD_moderate‐to‐severe CT = MDD_no or low CT > HC_moderate‐to‐severe CT = HC_no or low CT; HAMD: MDD_moderate‐to‐severe CT = MDD_no or low CT > HC_moderate‐to‐severe CT = HC_no or low CT; CTQ_total: MDD_moderate‐to‐severe CT > HC_moderate‐to‐severe CT > MDD_no or low CT = HC_no or low CT; Physical neglect: MDD_moderate‐to‐severe CT = HC_moderate‐to‐severe CT > MDD_no or low CT = HC_no or low CT; Physical abuse: MDD_moderate‐to‐severe CT > MDD_no or low CT = HC_no or low CT; Emotional neglect: MDD_moderate‐to‐severe CT > HC_moderate‐to‐severe CT > MDD_no or low CT = HC_no or low CT; Emotional abuse: MDD_moderate‐to‐severe CT > MDD_no or low CT = HC_no or low CT; Sexual abuse: HC_moderate‐to‐severe CT > HC_no or low CT.
Abbreviations: MDD, major depressive disorder; HC, healthy controls; HAMD, Hamilton Depression Rating Scale; PSS, Perceived Stress Scale; CTQ, Childhood Trauma Questionnaire; FD, frame displacement.
2.3.3. Seed definition and FC analysis
After preprocessing, seed‐based whole‐brain FC maps were calculated for each participant. Seeds were located in the striatum and the amygdala. Based on previous research (Di Martino et al., 2008), the six bilateral striatal seeds were defined as spheres with 4‐mm radius centered on the following coordinates (according to the MNI): NAcc (±9, 9, −8), ventral caudate (±10, 15, 0), dorsal caudate (±13, 15, 9), dorsal caudal putamen (±28, 1, 3), dorsal rostral putmen (±25, 8, 6), and ventral rostral putamen (±20, 12, −3). These striatal seeds were chosen due to their critical role in reward circuitry (Di Martino et al., 2008; Gabbay et al., 2013; Lin et al., 2017; Porter et al., 2015). The amygdala seeds were defined as spheres with a 4‐mm radius centered on the coordinates (MNI) of left amygdala (−24, −7, −26) and right amygdala (23, −7, −27) (Birn, Patriat, Phillips, Germain, & Herringa, 2014).
2.4. Statistical analysis
One‐way ANOVAs, two sample t‐tests and χ2 tests were used to evaluate the demographic and clinical differences among the four groups: MDD patients with moderate‐to‐severe CT (MDD_moderate‐to‐severe CT), MDD patients with no or low CT (MDD_no or low CT), HC with moderate‐to‐severe CT (HC_moderate‐to‐severe CT) and HC with no or low CT CT (HC_no or low CT).
Main effects of diagnosis, CT and the interaction between diagnosis and CT on depression symptomatology and anxiety level were analyzed by 2 (diagnosis: MDD, HC) × 2 (CT: moderate‐to‐severe level, no or low level) ANCOVAs. Depression symptomatology included different aspects of anhedonia, nonanhedonic depression and overall depression severity. Demographic and clinical variables that differed among four groups, that is, age in the present study, were controlled as covariates. The recent stress level was additionally controlled when investigating the effects involving CT.
Similarly, FC images generated by each seed, were analyzed by 2 (diagnosis: MDD, HC) × 2 (CT: moderate‐to‐severe level, no or low level) ANCOVAs. Demographic and clinical variables that differed among the four groups (i.e., age in the present study) and additional mean FD were controlled as covariates. Recent stress level was controlled when investigating the effects involving CT. Analyses were performed with SPM12. For the FCs based on striatal seeds, the comparison results were masked to a core frontal‐striatal reward circuitry described in previous research (Cha et al., 2016; Haber & Knutson, 2010; Liu et al., 2011), which includes the targets of medial and lateral OFC and ACC. The OFC included the following regions: left and right orbital superior/middle/inferior/medial frontal gyrus (Automated Anatomical Labeling map labels; 2,111, 2,112, 2,211, 2,212, 2,321, 2,322, 2,611, 2,612), and left and right rectus (2,701, 2,702), and the ACC was defined as: left and right ACC (4,001, 4,002) (Kahnt & Tobler, 2017). For the FCs from the seeds of amygdala, comparison results were checked both in the mask of frontal‐striatal reward circuitry and the whole brain. The significance threshold for all image comparisons was set at cluster‐level p < .05, a family‐wise error (FWE) corrected for multiple comparisons, starting from an uncorrected p value of .001 at the voxel level.
Where significant effects of CT on anhedonia and reward‐related FC were established, correlation analyses were carried out to examine whether these altered FCs were exclusively related to anhedonia in both MDD and HC respectively. If the correlations were established, the Haye's bootstrapping method (PROCESS macro based on SPSS; model 4, utilizing 5,000 bootstrap samples to estimate the 95% confidence interval) was performed to test the mediation models of reward‐related FC alterations on the relationship between CT and anhedonia symptomatology in both groups. Notably, if two altered FCs were found to be associated with one anhedonia component, including them both in one mediation model might introduce potential problem of multicollinearity due to the high correlation between FCs. To overcome this issue, a principal components analysis was conducted (Herringa et al., 2013). Briefly, this method would specify two independent (uncorrelated) components. The first component was calculated by summing the two FCs, which reflected the total or shared altered connectivity within reward system. The second component was defined as the differences between FCs, which reflected the differential connectivity. In addition, because the timing in the assessment of anhedonia and FC overlapped, the mediation models were also conducted with the positions of anhedonia and related FCs reversed.
3. RESULTS
3.1. Demographical and clinical variables
Gender distribution and education level did not differ among groups. However, groups differed in age. MDD_moderate‐to‐severe CT group was older than both HC groups. MDD_no or low CT group was older than HC_moderate‐to‐severe CT group. Two patient groups, and two HC groups had similar age with each other. Both patient groups scored higher on PSS compared to HC groups. PSS scores did not differ between two patient groups and two HC groups. The two patient groups were also similar in age onset, illness duration and overall illness severity. The four groups did not differ in the head motion parameters including the mean FD and the percentage of censored frames (Table 1).
3.2. Effects of CT on anhedonia
There were main effects of diagnosis on all depression symptomatology and anxiety measures, with MDD patients demonstrating significantly higher depression, anhedonia and anxiety level than HC. Main effects of CT were detected on measures of different components of anhedonia including PAS, SAS and anticipatory subscale of TEPS scores, indicating that participants with moderate‐to‐severe CT, independent of MDD diagnosis, demonstrated higher levels of physical, social, anticipatory but not consummatory trait anhedonia than participants with no or low exposure of CT. Significant main effect of CT was also revealed on state anhedonia, however, this main effect was further qualified by a significant interaction of diagnosis by CT, revealing that only in MDD group, participants with moderate‐to‐severe CT suffered more severe state anhedonia than participants with no or low exposure. No significant main effects of CT or CT by diagnosis interactions on anxiety, nonanhedonic depression, overall depression level and consummatory anhedonia were detected (Table 2).
TABLE 2.
Diagnosis and childhood trauma effects on anxiety, depression and anhedonia
| Characteristics | MDD | HC | ANCOVA results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate‐to‐severe CT | No or low CT | Moderate‐to‐severe CT | No or low CT | Effect of diagnosis | Effect of CT | Effect of diagnosis x CT | |||||||
| (N = 44) | (N = 22) | (N = 23) | (N = 34) | F | p | Eta2 | F | p | Eta2 | F | p | Eta2 | |
| PAS | 32.27 (11.79) | 24.95 (13.58) | 18.70 (6.69) | 13.79 (6.99) | 26.02 | <.001 | 0.18 | 5.62 | <.05 | 0.05 | 0.28 | .596 | — |
| SAS | 22.55 (6.98) | 18.27 (7.96) | 12.83 (4.31) | 8.97 (4.39) | 50.46 | <.001 | 0.30 | 8.12 | <.01 | 0.07 | 0.02 | .898 | — |
| TEPS_ANT | 30.32 (9.39) | 35.36 (8.76) | 39.39 (5.00) | 40.79 (6.41) | 13.11 | <.001 | 0.10 | 4.40 | <.05 | 0.04 | 1.02 | .314 | — |
| TEPS_CON | 33.52 (10.17) | 33.95 (10.02) | 39.83 (5.83) | 41.15 (8.52) | 8.14 | <.01 | 0.07 | 0.05 | .822 | — | 0.19 | .665 | — |
| BDI_anhedonia | 4.45 (1.58) | 3.05 (1.76) | 1.26 (1.10) | 0.91 (1.08) | 75.29 | <.001 | 0.41 | 5.71 | <.05 | 0.05 | 4.43 | <.05 a | 0.04 |
| BDI_noanhedonia | 26.80 (9.12) | 22.05 (9.33) | 7.35 (6.12) | 4.47 (3.80) | 152.41 | <.001 | 0.51 | 1.24 | .267 | — | 1.55 | .216 | — |
| HAMD | 21.68 (6.55) | 20.50 (6.69) | 1.22 (1.68) | 1.03 (1.80) | 363.92 | <.001 | 0.76 | 0.23 | .632 | — | 0.22 | .637 | — |
| STAI_S | 60.23 (12.73) | 54.55 (11.41) | 39.70 (8.92) | 35.79 (8.60) | 81.34 | <.001 | 0.41 | 0.71 | .400 | — | 0.66 | .417 | — |
| STAI_T | 63.20 (8.72) | 58.82 (7.73) | 44.13 (7.82) | 39.79 (9.04) | 123.96 | <.001 | 0.51 | 1.15 | .286 | — | 0.15 | .701 | — |
Note: Means with standard deviations in parentheses.
Abbreviations: BDI, Beck Depression Inventory; BDI_anhedonia, sum of item 4, 12 and 21 in BDI; BDI_noanhedonia, sum of BDI items excluded item 4, 12 and 21; CT, Childhood Trauma; HAMD, Hamilton Depression Rating Scale; HC, healthy controls; MDD, major depressive disorder patients; PAS, Physical Anhedonia Scale; SAS, Social Anhedonia Scale; STAI_S, Spielberger Stait‐Trait Anxiety Inventory_State Form; STAI_T, Spielberger Stait‐Trait Anxiety Inventory_Trait Form; TEPS, Temporal Experience of Pleasure Scale; TEPS_ANT, anticipatory subscale of the TEPS; TEPS_CON, consummatory subscale of the TEPS.
Further simple effects analysis revealed that only in MDD group, participants with moderate‐to‐severe CT demonstrated higher level of state anhedonia than participants with no or low CT (MDD_moderate‐to‐severe CT vs. MDD_no or low CT: p < 0.005; HC_moderate‐to‐severe CT vs. HC_no or low CT: p = .811).
3.3. Effects of CT on FC
For FCs from the striatal seeds, there were significant main effects of CT. Participants with moderate‐to‐severe exposure, independent of MDD diagnosis showed decreased FC between left NAcc seed and right orbital middle frontal gyrus and between left ventral caudate seed and left orbital frontal gyrus (p < .05 FWEcorr‐cluster). There were main effects of diagnosis. MDD patients had decreased FC between right dorsal caudate seed and right ACC (p < .05 FWEcorr‐cluster). No other significant main effects or interactions of diagnosis by CT on FC were detected (Table 3, Figure 1a,d,df. For FCs based on the amygdale seeds, we failed to detect any significant main effects of CT, diagnosis or interactions of diagnosis by CT.
TABLE 3.
Diagnosis and childhood trauma effects on functional connectivity within reward system based on the striatum seeds
| Seeds | Brain regions | Voxel | Peak coordinates (x/y/z; MNI) | Peak T values | p‐value (FWEcorr‐cluster) | |||
|---|---|---|---|---|---|---|---|---|
| With moderate‐to‐severe CT < with no or low CT | ||||||||
| Left NAcc | Right orbital middle frontal gyrus (BA 10, 47) | 22 | 36 | 60 | −3 | 4.46 | .023 | |
| Left ventral caudate | Left orbital inferior and middle frontal gyrus (BA 11) | 21 | −30 | 42 | −18 | 3.91 | .023 | |
| MDD < HC | ||||||||
| Right dorsal caudate | Right anterior cingulate cortex (BA32) | 20 | 9 | 33 | 24 | 4.28 | .026 | |
Note: 1 subject did not perform the fMRI scan, and the data of 2 participants in the MDD group (1 MDD_moderate‐to‐severe CT, 1 MDD_no or low CT) were excluded from the imaging analyses. Thus, the results of image analyses and subsequent correlation and mediation analyses were based on the sample of 42 MDD_moderate‐to‐severe CT and 21 MDD_no or low CT.
Abbreviations: MDD, patients with major depressive disorder; HC, healthy controls; CT, childhood trauma; BA, Broadmann area; x, y, z, coordinates of peak locations in the Montreal Neurological Institute space (MNI). p < .05, cluster‐level FWE corrected with voxel‐level starting from p < .001 uncorrected.
FIGURE 1.
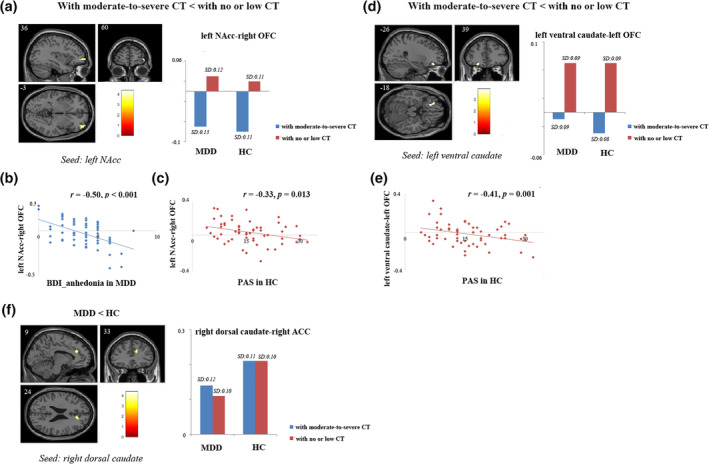
Results of image analyses and correlation analyses. (a) Participants with a history of moderate‐to‐severe CT showed reduced FC between the left NAcc seed and the right OFC compared to participants with no or low level of CT. The altered connectivity between left NAcc and right OFC was significantly correlated with (b) BDI_anhedonia scores in MDD (r = −0.50, p < .001) and (c) physical trait anhedonia scores in HC (r = −0.33, p = .013); (d) Participants with a history of moderate‐to‐severe CT showed decreased FC between the left ventral caudate seed and the left OFC compared to participants with no or low level of CT; (e) The altered connectivity between left ventral caudate and left OFC was significantly correlated with physical trait anhedonia scores in HC (r = −0.41, p = .001); (f) MDD patients had reduced FC between right dorsal caudate and right ACC compared to HC. For image analyses, results were restricted to a mask combining OFC and ACC ROIs. Significance threshold was set at p < .05, FWE cluster level corrected, starting from voxel level p < .001 uncorrected. ACC, anterior cingulate cortex; BDI_anhedonia, sum of anhedonia‐related item 4, 12, and 21 in BDI; CT, childhood trauma; CTQ, childhood trauma questionnaire; FC, functional connectivity; HC, healthy controls; MDD, major depressive disorder; NAcc, nucleus accumbens; OFC, orbital frontal gyrus; PAS, Physical Anhedonia Scale; PSS, Perceived Stress Scale
3.4. Correlation and mediation analyses
Results showed that the connectivity between left NAcc and right OFC was significantly correlated with state anhedonia in MDD group (r = −0.50, p < .001) (Figure 1(b)). Mediation analysis revealed that in MDD, the mediation effect of left NAcc‐right OFC FC on the relationship between CT and state anhedonia (β = 0.13, bootstrapped 95% CI = 0.0044 to 0.0368) was significant, and after controlling for FC mediation effects, the direct effects of CT on state anhedonia were no longer significant (β = 0.11, bootstrapped 95% CI = −0.0161 to 0.0429) (Figure 2(a)).
FIGURE 2.
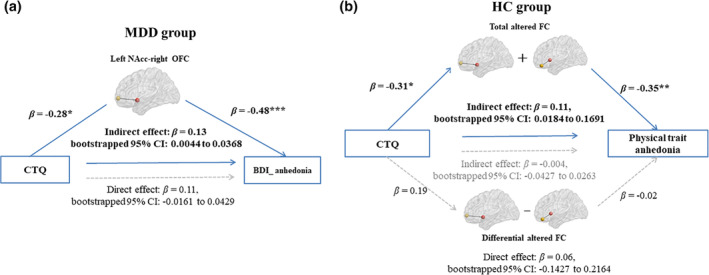
Mediation models for effects of striatum‐based FC within core reward system on the relationship between CT and anhedonia in MDD and HC. (a) FC between the left NAcc and the right OFC significantly mediated the relationship between CT and state anhedonia in MDD; (b) Total altered FCs between the left NAcc and the right OFC and between left ventral caudate and left OFC significantly mediated the relationship between CT and physical trait anhedonia in HC. Mediation analyses were generated by using the bootstrap method from 5,000 bootstrapped samples. *, p < .05; **, p < .01; ***, p < .001; BDI_anhedonia, sum of anhedonia‐related item 4, 12 and 21 in BDI; CT, childhood trauma; CTQ, childhood trauma questionnaire; Differential FC, left NAcc‐the right OFC minus left ventral caudate‐left OFC; FC, functional connectivity; HC, healthy controls; β, standardized coefficient; CI, confidence interval
Both the altered FCs of left NAcc‐ right OFC (r = −0.33, p = .013) and left ventral caudate‐left OFC (r = −0.41, p = .001) were significantly correlated with physical trait anhedonia in HCs (significant with Bonferroni correction [0.05/3]; Figure 1c,e). Because both the FC of left NAcc‐right OFC and left ventral caudate‐left OFC were significantly correlated with physical anhedonia, and the two FCs were highly correlated (r = 0.53, p < .001), we further constructed two independent (uncorrelated) measures: the sum of the FCs and the differences between FCs (r = −0.05, p = .690) to avoid the potential problem of multicolinearity. Results showed that the total indirect effects of CT on physical trait anhedonia was significant (β = 0.10, bootstrapped 95% CI = 0.0004 to 0.1607), driven by mediatory effect of the total altered connectivity (β = 0.11, bootstrapped 95% CI = 0.0184 to 0.1691) while not the differential connectivity (β = −0.004, bootstrapped 95% CI = −0.0427 to 0.0263). After controlling for the indirect effects, direct effects of CT on physical trait anhedonia in HCs was not significant (β = 0.06, bootstrapped 95% CI = −0.1427 to 0.2164) (Figure 2b).
Reversal of these two mediation models (CT‐anhedonia‐FC) in MDD and HC yield nonsignificant results (MDD: bootstrapped 95% CI = −0.0031 to 0.0000; HC: bootstrapped 95% CI = −0.0040 to 0.0004). No other significant correlations between altered FCs and anhedonia as well as no‐anhedonia symptoms were detected ( Table S1 ).
4. DISCUSSION
This study investigated the effects of CT on different aspects of anhedonia via the reward system. In addition to generally greater depression and anxiety in MDD compared with HC, the following interesting findings were revealed: (a) MDD patients with moderate‐to‐severe CT had elevated state anhedonia compared with MDD patients with no or low CT; (b) CT has effects on specific types of trait anhedonia and on core reward system; (c) FC of left NAcc‐right OFC within reward system mediated the relationship between CT and state anhedonia in MDD; (d) total altered FC of left NAcc‐right OFC and left ventral caudate‐left OFC mediated the links between CT and physical trait anhedonia in HCs. These findings highlight the specific aspects of anhedonia and the alterations of VS‐OFC connectivity as targets of CT, and support CT‐reward related FC alterations‐anhedonia may be manifested as important mechanisms involved in depression following CT.
Our behavioral results revealed a significant interaction of diagnosis by CT on state anhedonia, indicating that MDD patients with moderate or greater CT had more severe state anhedonia than MDD patients with no or low level of CT. Subsequent imaging, correlation and mediation analyses revealed that FC between NAcc and OFC mediated the relationship between CT and state anhedonia in MDD patients. The findings of CT's effects only on anhedonia but not on anxiety and nonanhedonic depression were in line with our hypothesis, suggesting that CT may mainly have effects on anhedonia aspect of MDD. This finding was in agreement with several prior animal studies (Bai et al., 2014; Molet et al., 2016), while it is not consistent with Dillon et al. (2009), which found that subjects with CT had not only higher anhedonia but also general depression level than HCs without CT. However, Dillon et al. (2009) only included 13 subjects with CT and several subjects among the 13 had past MDD or anxiety disorders. The small sample size as well as the confounding effects of past psychiatric disorders may hinder the generalization of their results.
NAcc is part of the VS, which along with the OFC are involved in the core reward system (Liu et al., 2011). Hence, our findings further suggest that CT affects anhedonic depression via FC within the core reward system, which may be involved in the pathogenesis of MDD with CT. NAcc and OFC are both major dopaminergic projection areas (Nestler & Carlezon Jr., 2006). Thus, these results may highlight the involvement of dopamine system in CT leading to MDD. Combined with the ANCOVA findings which revealed MDD patients with CT characterized by more severe anhedonia and more VS‐OFC alterations, these results may further suggest that MDD with CT represent a specific MDD subtype with unique etiology (involved CT), pathological mechanisms (more dopamine system involved) as well as clinical presentations (more anhedonia), strengthening the insight into the heterogeneity of MDD. Interestingly, previous studies revealed that MDD with CT is associated with unfavorable responses to pharmacological interventions (Tunnard et al., 2014). Reward‐circuit connectivity and anhedonia in MDD were also correlated with the unresponsive to treatment (Downar et al., 2014; McMakin et al., 2012). So far, most existing antidepressant medications target the serotonergic or noradrenergic systems (Nutt, 2002). Thus, our results raise the possibility that the relatively low treatment response in MDD patients with CT may be due to a lack of medication targeting the dopaminergic system. In the present study, the elevated state anhedonia in individuals with more severe level of CT was not observed in HC, suggesting that the degree of state anhedonia difference between two MDD groups and between two HC groups were different. Different trauma characteristics between MDD_moderate‐to‐severe CT and HC_moderate‐to‐severe CT may be partly account for this finding. In the present study, from the view of specific type of trauma, MDD_moderate‐to‐severe CT group mainly had greater emotional neglect/abuse compared with HC_moderate‐to‐severe CT group. Correlation analysis revealed that though state anhedonia was significantly corelated with CTQ total score, the stronger association came from specific trauma type of emotional neglect ( Table S2 ). Hence, less significant difference of emotional neglect in HC_moderate‐to‐severe CT may partly help explain the negative comparison results of state anhedonia between two HC groups. Future research with big sample size and longitudinal design can be conducted to investigate the common and specific mechanisms via which different CT types lead to depression.
Besides state anhedonia, our results also revealed that CT had main effects on specific type of trait anhedonia. Specifically, we found that individuals with moderate‐to‐severe CT, irrespective of MDD diagnosis, had higher physical, social, anticipatory but not consummatory trait anhedonia than participants with no or low level of CT. Physical and social anhedonia was distinguished on the basis of the contents that engender pleasure. Anticipatory and consummatory anhedonia was differentiated according to the cognitive phase that the pleasure produces. Different from consummatory pleasure, which is the in‐the‐moment experience responding to pleasurable stimulus, the anticipatory pleasure can be described as a feeling of “wanting” and is related to goal‐directed motivation and expected reward (Gard et al., 2006). CT has effects on physical, social and anticipatory but not consummatory anhedonia indicate that the anhedonia affected by CT may be general to all types of rewards, while specific to certain cognitive stages: CT may not affect the ability to experience pleasure to rewards in real time, while it may hinder the ability to anticipate pleasure. To our knowledge, no study has used the TEPS to compare the effects of CT on anticipatory and consummatory anhedonia directly. However, several studies have found that individuals experienced CT had reduced brain activation during anticipation but not outcome (consummatory) phase in the reward‐related cognitive tasks (Casement et al., 2014; Dillon et al., 2009; Mehta et al., 2010; Romens et al., 2015), which support our findings.
CT's main effect was also revealed on FC between left NAcc and the right OFC and between left ventral caudate and the left OFC, suggesting that connectivity of the VS‐OFC within the core reward circuit is a target of CT in both MDD and HC. These results are consistent with previous studies demonstrating that CT is associated with altered VS activity during specific tasks, and with OFC volume (Chaney et al., 2014; Goff et al., 2013; Mehta et al., 2010). Our mediation analyses further revealed that the total altered FC of left NAcc‐right OFC and left ventral‐left OFC significantly mediated the relationship between CT and physical trait anhedonia in HC. These findings help link the neural alterations companied with CT to the behavioral targets. Effects of CT on trait anhedonia and VS‐OFC connectivity were similarly existed in MDD and HC. Previous studies have revealed that anhedonia following CT can be observed as early as in periweaning, enduring to later‐life and followed by depressive‐like behaviors (Raineki, Cortes, Belnoue, & Sullivan, 2012; Rincon‐Cortes & Sullivan, 2016). Human longitudinal studies have showed depression prediction in anhedonia and brain reward system (Casement et al., 2014; Hanson et al., 2015; Romens et al., 2015). These findings raise the possibility that effects of CT on trait‐like anhedonia and reward‐related FC found in the present study may be in relation to future MDD. However, due to the cross‐sectional design of the present study, we can only speculate while cannot assert this possibility. In addition, our similar alterations of VS‐OFC connectivity in MDD_CT an HC_CT may be at first sight, seem to challenge the mechanism of why some individuals developed MDD. It should be noted that in recent years, main effects of CT while not CT and diagnosis interactions were not novel findings. Several prior studies have also shown an effect of CT on brain structure and function, independent of MDD diagnosis (Chaney et al., 2014; Lu et al., 2019; Meinert et al., 2019; Opel et al., 2014; van Harmelen et al., 2010), indicating that some brain alterations previously attributed to MDD diagnosis might rather be characterized as a function of maltreatment. These findings might be consistent with the notion that additional risk factors, such as genetic makeup alone or in interaction with exposure to stressful life events during adulthood may additionally determine who will subsequently develop MDD (Caspi & Moffitt, 2006; Frodl et al., 2010; Gatt et al., 2009; Joffe et al., 2009). Nevertheless, if some alterations were more likely to be attributed to CT, the question of why some individuals might develop MDD needs to be examined by future longitudinal design, which takes more factors into consideration, and this is our next step of research.
Besides effects involving CT, our study also revealed main effect of diagnosis on FC in reward system, indicating that MDD patients generally have reduced FC between right dorsal caudate seed and right ACC relative to HC. We failed to reveal the significant relationship between this FC and any of types of anhedonia examined in the present study (Table S1). Previous studies have shown that both dorsal caudate and ACC were involved in many reward‐related processes, such as reward learning and reward‐related decision‐making (Kennerley, Walton, Behrens, Buckley, & Rushworth, 2006; Rushworth & Behrens, 2008; Seo & Lee, 2007). These results may suggest that the reduced FC between right dorsal caudate and right ACC indicate that MDD patients have deficits in these two reward‐related function domains, which cannot be well captured by our anhedonia facet measures. In addition, we failed to detect any effects of CT or interactions of diagnosis by CT on the FCs based on the amygdala seeds. These findings indicate that amygdala may be not involved in the mechanism of anhedonia following CT. However, this suggestion should be treated with caution as our results also not replicated previous findings of CT having effects on amygdala activity through the involvement in fear circuit (Dannlowski et al., 2012; Herringa et al., 2013; Van der Werff et al., 2013), and we failed to detect the main effect of diagnosis on amygdala‐based FCs. Further studies with big sample size may be needed to confirm the role of amygdala in the relationship between CT and depression.
Several limitations of the present study should be addressed. First, the CTQ is completed retrospectively, and the scores of which might be influenced by the inherent subjectivity and/or the current mood state of the participants. Future prospective studies may be able to overcome this issue. Second, due to the cross‐sectional nature of the study, the determination of causality needs to be cautious. Also, the role of elevated trait anhedonia and decreased FC within reward system found in individuals with moderate‐to‐severe CT in the development of future MDD needs to be investigated in studies with longitudinal design. Third, the MDD and HC was not well‐matched on age. However, age was controlled as a covariate in the comparison analyses. The additional analysis failed to reveal any significant correlations between the altered FC and age ( Table S3 ). Also, the age was not differed between two patient groups and two HC groups. These all suggest that our main results, that is, the main effects of CT, were not likely driven by age differences. Fourth, in both groups, the increases in social trait anhedonia related to CT cannot be explained by alterations in core reward circuitry. It may be that other brain system, such as systems involved in the social cognition play a role in the links between CT and social anhedonia. Future studies can examine the impact of CT on brain regions subserving social cognition functioning and the relationship with social trait anhedonia.
5. CONCLUSION
In summary, this study demonstrated that the specific aspects of anhedonia, that is, physical, social, anticipatory but not consummatory anhedonia and connectivity of VS‐OFC within reward system were targets of CT. A direct mechanism, via the connectivity between the left NAcc and right OFC within the reward circuit mediated the relationship between CT and state anhedonia in MDD. These findings may have implication for mechanism of CT leading to depression and suggest the need to investigate treatments targeting dopamine system in MDD patients exposed to CT.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (Xiongzhao Zhu, grant number 81671341; Changlian Tan, grant number 81471646), and the Key Laboratory of Mental Healthy, Institute of Psychology, Chinese Academy of Science (Xiongzhao Zhu, grant number KLMH2015K01). We are grateful for the generosity of time and effort by all the participants, and all researchers who make this project possible.
Fan J, Liu W, Xia J, et al. Childhood trauma is associated with elevated anhedonia and altered core reward circuitry in major depression patients and controls. Hum Brain Mapp. 2021;42:286–297. 10.1002/hbm.25222
Funding information National Natural Science Foundation of China, Grant/Award Number: 81671341; the Key Laboratory of Mental Healthy, Institute of Psychology, Chinese Academy of Science, Grant/Award Number: KLMH2015K01; National Natural Science Foundation of China, Grant/Award Number: 81471646
Contributor Information
Changlian Tan, Email: tanchanglian@csu.edu.cn.
Xiongzhao Zhu, Email: xiongzhaozhu@csu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of the study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Agrawal, A. , Nelson, E. C. , Littlefield, A. K. , Bucholz, K. K. , Degenhardt, L. , Henders, A. K. , … Lynskey, M. T. (2012). Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Archives of General Psychiatry, 69(7), 732–740. 10.1001/archgenpsychiatry.2011.2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, M. , Zhang, L. , Zhu, X. , Zhang, Y. , Zhang, S. , & Xue, L. (2014). Comparison of depressive behaviors induced by three stress paradigms in rats. Physiology & Behavior, 131, 81–86. 10.1016/j.physbeh.2014.04.019 [DOI] [PubMed] [Google Scholar]
- Ballard, E. D. , Wills, K. , Lally, N. , Richards, E. M. , Luckenbaugh, D. A. , Walls, T. , … Zarate, C. A., Jr. (2017). Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. Journal of Affective Disorders, 218, 195–200. 10.1016/j.jad.2017.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. (1965). An inventory for measuring depression. Archives of General Psychiatry, 12, 63–70. [DOI] [PubMed] [Google Scholar]
- Bernstein, D. P. , & Fink, L. (1998). Childhood trauma questionnaire: A retrospective self‐report manual. San Antonio: Harcourt Brace & Company. [Google Scholar]
- Bernstein, D. P. , Fink, L. , Handelsman, L. , Foote, J. , Lovejoy, M. , Wenzel, K. , … Ruggiero, J. (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry, 151(8), 1132–1136. 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- Birn, R. M. , Patriat, R. , Phillips, M. L. , Germain, A. , & Herringa, R. J. (2014). Childhood maltreatment and combat posttraumatic stress differentially predict fear‐related fronto‐subcortical connectivity. Depression and Anxiety, 31(10), 880–892. 10.1002/da.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker, R. , Holz, N. E. , Buchmann, A. F. , Blomeyer, D. , Plichta, M. M. , Wolf, I. , . . . Laucht, M. (2014). Impact of early life adversity on reward processing in young adults: EEG‐fMRI results from a prospective study over 25 years. PLoS One , 9(8), e104185 10.1371/journal.pone.0104185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, J. L. , Molet, J. , Regev, L. , Chen, Y. , Rismanchi, N. , Haddad, E. , … Baram, T. Z. (2018). Anhedonia following early‐life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala Corticotropin‐releasing hormone gene. Biological Psychiatry, 83(2), 137–147. 10.1016/j.biopsych.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement, M. D. , Guyer, A. E. , Hipwell, A. E. , McAloon, R. L. , Hoffmann, A. M. , Keenan, K. E. , & Forbes, E. E. (2014). Girls' challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience, 8, 18–27. 10.1016/j.dcn.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi, A. , & Moffitt, T. E. (2006). Gene‐environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews. Neuroscience, 7(7), 583–590. 10.1038/nrn1925 [DOI] [PubMed] [Google Scholar]
- Cha, J. , Ide, J. S. , Bowman, F. D. , Simpson, H. B. , Posner, J. , & Steinglass, J. E. (2016). Abnormal reward circuitry in anorexia nervosa: A longitudinal, multimodal MRI study. Human Brain Mapping, 37(11), 3835–3846. 10.1002/hbm.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney, A. , Carballedo, A. , Amico, F. , Fagan, A. , Skokauskas, N. , Meaney, J. , & Frodl, T. (2014). Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. Journal of Psychiatry & Neuroscience, 39(1), 50–59. 10.1503/jpn.120208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, L. J. , Chapman, J. P. , & Raulin, M. L. (1976). Scales for physical and social anhedonia. Journal of Abnormal Psychology, 85(4), 374–382. [DOI] [PubMed] [Google Scholar]
- Chapman, L. J. , & Chapman, L. P. (1978). Revised physical anhedonia scale. Unpublished Test, 476–489.
- Cohen, A. S. , Najolia, G. M. , Brown, L. A. , & Minor, K. S. (2011). The state‐trait disjunction of anhedonia in schizophrenia: Potential affective, cognitive and social‐based mechanisms. Clinical Psychology Review, 31(3), 440–448. 10.1016/j.cpr.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Cohen, S. , Kamarck, T. , & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Costa Dias, T. G. , Iyer, S. P. , Carpenter, S. D. , Cary, R. P. , Wilson, V. B. , Mitchell, S. H. , … Fair, D. A. (2015). Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Developmental Cognitive Neuroscience, 11, 155–174. 10.1016/j.dcn.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash, O. , Fornito, A. , Lee, J. , Keefe, R. S. , Chee, M. W. , Adcock, R. A. , … Harrison, B. J. (2014). Altered striatal functional connectivity in subjects with an at‐risk mental state for psychosis. Schizophrenia Bulletin, 40(4), 904–913. 10.1093/schbul/sbt093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski, U. , Stuhrmann, A. , Beutelmann, V. , Zwanzger, P. , Lenzen, T. , Grotegerd, D. , … Kugel, H. (2012). Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- Di Martino, A. , Scheres, A. , Margulies, D. S. , Kelly, A. M. , Uddin, L. Q. , Shehzad, Z. , … Milham, M. P. (2008). Functional connectivity of human striatum: A resting state FMRI study. Cerebral Cortex, 18(12), 2735–2747. 10.1093/cercor/bhn041 [DOI] [PubMed] [Google Scholar]
- Dillon, D. G. , Holmes, A. J. , Birk, J. L. , Brooks, N. , Lyons‐Ruth, K. , & Pizzagalli, D. A. (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry, 66(3), 206–213. 10.1016/j.biopsych.2009.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Li, H. , Wang, Y. , & Potenza, M. N. (2018). Individual differences in self‐reported reward‐approach tendencies relate to resting‐state and reward‐task‐based fMRI measures. International Journal of Psychophysiology, 128, 31–39. 10.1016/j.ijpsycho.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Downar, J. , Geraci, J. , Salomons, T. V. , Dunlop, K. , Wheeler, S. , McAndrews, M. P. , … Giacobbe, P. (2014). Anhedonia and reward‐circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biological Psychiatry, 76(3), 176–185. 10.1016/j.biopsych.2013.10.026 [DOI] [PubMed] [Google Scholar]
- Drysdale, A. T. , Grosenick, L. , Downar, J. , Dunlop, K. , Mansouri, F. , Meng, Y. , … Liston, C. (2017). Resting‐state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine, 23(1), 28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad, M. L. , Chapman, L. J. , Chapman, J. P. , & Mishlove, M. (1982). The revised social anhedonia scale. Unpublished test.
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience, 8(9), 700–711. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Frodl, T. , Reinhold, E. , Koutsouleris, N. , Donohoe, G. , Bondy, B. , Reiser, M. , … Meisenzahl, E. M. (2010). Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology, 35(6), 1383–1390. 10.1038/npp.2010.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay, V. , Ely, B. A. , Li, Q. , Bangaru, S. D. , Panzer, A. M. , Alonso, C. M. , … Milham, M. P. (2013). Striatum‐based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry, 52(6), 628–641 e613. 10.1016/j.jaac.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard, D. E. , Gard, M. G. , Kring, A. M. , & John, O. P. (2006). Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality, 40(6), 1086–1102. [Google Scholar]
- Gatt, J. M. , Nemeroff, C. B. , Dobson‐Stone, C. , Paul, R. H. , Bryant, R. A. , Schofield, P. R. , … Williams, L. M. (2009). Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry, 14(7), 681–695. 10.1038/mp.2008.143 [DOI] [PubMed] [Google Scholar]
- Germine, L. , Dunn, E. C. , McLaughlin, K. A. , & Smoller, J. W. (2015). Childhood adversity is associated with adult theory of mind and social affiliation, but not face processing. PLoS One, 10(6), e0129612 10.1371/journal.pone.0129612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff, B. , Gee, D. G. , Telzer, E. H. , Humphreys, K. L. , Gabard‐Durnam, L. , Flannery, J. , & Tottenham, N. (2013). Reduced nucleus accumbens reactivity and adolescent depression following early‐life stress. Neuroscience, 249, 129–138. 10.1016/j.neuroscience.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse, L. , Ambree, O. , Jorgens, S. , Jawahar, M. C. , Singhal, G. , Stacey, D. , … Baune, B. T. (2016). Cytokine levels in major depression are related to childhood trauma but not to recent stressors. Psychoneuroendocrinology, 73, 24–31. 10.1016/j.psyneuen.2016.07.205 [DOI] [PubMed] [Google Scholar]
- Haber, S. N. , & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Albert, D. , Iselin, A. M. , Carre, J. M. , Dodge, K. A. , & Hariri, A. R. (2016). Cumulative stress in childhood is associated with blunted reward‐related brain activity in adulthood. Social Cognitive and Affective Neuroscience, 11(3), 405–412. 10.1093/scan/nsv124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Hariri, A. R. , & Williamson, D. E. (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78(9), 598–605. 10.1016/j.biopsych.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa, R. J. , Birn, R. M. , Ruttle, P. L. , Burghy, C. A. , Stodola, D. E. , Davidson, R. J. , & Essex, M. J. (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America, 110(47), 19119–19124. 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe, R. T. , Gatt, J. M. , Kemp, A. H. , Grieve, S. , Dobson‐Stone, C. , Kuan, S. A. , … Williams, L. M. (2009). Brain derived neurotrophic factor Val66Met polymorphism, the five factor model of personality and hippocampal volume: Implications for depressive illness. Human Brain Mapping, 30(4), 1246–1256. 10.1002/hbm.20592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner, T. E. , Brown, J. S. , & Metalsky, G. I. (2003). A test of the tripartite model's prediction of anhedonia's specificity to depression: Patients with major depression versus patients with schizophrenia. Psychiatry Research, 119(3), 243–250. [DOI] [PubMed] [Google Scholar]
- Kahnt, T. , & Tobler, P. N. (2017). Dopamine modulates the functional Organization of the Orbitofrontal Cortex. The Journal of Neuroscience, 37(6), 1493–1504. 10.1523/JNEUROSCI.2827-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley, S. W. , Walton, M. E. , Behrens, T. E. , Buckley, M. J. , & Rushworth, M. F. (2006). Optimal decision making and the anterior cingulate cortex. Nature Neuroscience, 9(7), 940–947. 10.1038/nn1724 [DOI] [PubMed] [Google Scholar]
- Li, N. , Ma, N. , Liu, Y. , He, X. S. , Sun, D. L. , Fu, X. M. , … Zhang, D. R. (2013). Resting‐state functional connectivity predicts impulsivity in economic decision‐making. The Journal of Neuroscience, 33(11), 4886–4895. 10.1523/JNEUROSCI.1342-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W. C. , Chen, H. L. , Hsu, T. W. , Hsu, C. C. , Huang, Y. C. , Tsai, N. W. , & Lu, C. H. (2017). Correlation between dopamine transporter degradation and Striatocortical network alteration in Parkinson's disease. Frontiers in Neurology, 8, 323 10.3389/fneur.2017.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips, M. A. , Wijngaarden, M. A. , van der Grond, J. , van Buchem, M. A. , de Groot, G. H. , Rombouts, S. A. , … Veer, I. M. (2014). Resting‐state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. The American Journal of Clinical Nutrition, 100(2), 524–531. 10.3945/ajcn.113.080671 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Hairston, J. , Schrier, M. , & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–1236. 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. , Xu, R. , Cao, J. , Yin, Y. , Gao, W. , Wang, D. , … Xu, Y. (2019). The left dorsolateral prefrontal cortex volume is reduced in adults reporting childhood trauma independent of depression diagnosis. Journal of Psychiatric Research, 112, 12–17. 10.1016/j.jpsychires.2019.02.014 [DOI] [PubMed] [Google Scholar]
- McMakin, D. L. , Olino, T. M. , Porta, G. , Dietz, L. J. , Emslie, G. , Clarke, G. , … Brent, D. A. (2012). Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment‐resistant depression. Journal of the American Academy of Child and Adolescent Psychiatry, 51(4), 404–411. 10.1016/j.jaac.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, M. A. , Gore‐Langton, E. , Golembo, N. , Colvert, E. , Williams, S. C. , & Sonuga‐Barke, E. (2010). Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience, 22(10), 2316–2325. 10.1162/jocn.2009.21394 [DOI] [PubMed] [Google Scholar]
- Meinert, S. , Repple, J. , Nenadic, I. , Krug, A. , Jansen, A. , Grotegerd, D. , … Dannlowski, U. (2019). Reduced fractional anisotropy in depressed patients due to childhood maltreatment rather than diagnosis. Neuropsychopharmacology, 44(12), 2065–2072. 10.1038/s41386-019-0472-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet, J. , Heins, K. , Zhuo, X. , Mei, Y. T. , Regev, L. , Baram, T. Z. , & Stern, H. (2016). Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Translational Psychiatry, 6, e702 10.1038/tp.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog, N. K. , Entringer, S. , Rasmussen, J. M. , Styner, M. , Gilmore, J. H. , Kathmann, N. , … Buss, C. (2018). Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biological Psychiatry, 83(2), 120–127. 10.1016/j.biopsych.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, E. A. (2007). The amygdala, reward and emotion. Trends in Cognitive Sciences, 11(11), 489–497. 10.1016/j.tics.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Nestler, E. J. , & Carlezon, W. A., Jr. (2006). The mesolimbic dopamine reward circuit in depression. Biological Psychiatry, 59(12), 1151–1159. 10.1016/j.biopsych.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Nutt, D. J. (2002). The neuropharmacology of serotonin and noradrenaline in depression. International Clinical Psychopharmacology, 17(Suppl 1), S1–S12. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Opel, N. , Redlich, R. , Zwanzger, P. , Grotegerd, D. , Arolt, V. , Heindel, W. , … Dannlowski, U. (2014). Hippocampal atrophy in major depression: A function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology, 39(12), 2723–2731. 10.1038/npp.2014.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine, D. S. , Cohen, E. , Cohen, P. , & Brook, J. (1999). Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? The American Journal of Psychiatry, 156(1), 133–135. 10.1176/ajp.156.1.133 [DOI] [PubMed] [Google Scholar]
- Porter, J. N. , Roy, A. K. , Benson, B. , Carlisi, C. , Collins, P. F. , Leibenluft, E. , … Ernst, M. (2015). Age‐related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Developmental Cognitive Neuroscience, 11, 83–95. 10.1016/j.dcn.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki, C. , Cortes, M. R. , Belnoue, L. , & Sullivan, R. M. (2012). Effects of early‐life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive‐like behaviors mediated by the amygdala. The Journal of Neuroscience, 32(22), 7758–7765. 10.1523/JNEUROSCI.5843-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon‐Cortes, M. , & Sullivan, R. M. (2016). Emergence of social behavior deficit, blunted corticolimbic activity and adult depression‐like behavior in a rodent model of maternal maltreatment. Translational Psychiatry, 6(10), e930 10.1038/tp.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens, S. E. , Casement, M. D. , McAloon, R. , Keenan, K. , Hipwell, A. E. , Guyer, A. E. , & Forbes, E. E. (2015). Adolescent girls' neural response to reward mediates the relation between childhood financial disadvantage and depression. Journal of Child Psychology and Psychiatry, 56(11), 1177–1184. 10.1111/jcpp.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth, M. F. , & Behrens, T. E. (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11(4), 389–397. 10.1038/nn2066 [DOI] [PubMed] [Google Scholar]
- Satterthwaite, T. D. , Kable, J. W. , Vandekar, L. , Katchmar, N. , Bassett, D. S. , Baldassano, C. F. , … Wolf, D. H. (2015). Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology, 40(9), 2258–2268. 10.1038/npp.2015.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, H. , & Lee, D. (2007). Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed‐strategy game. The Journal of Neuroscience, 27(31), 8366–8377. 10.1523/JNEUROSCI.2369-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris, A. , Vidal‐Ribas Belil, P. , Artiges, E. , Lemaitre, H. , Gollier‐Briant, F. , Wolke, S. , … Consortium, I. (2015). The Brain's response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community‐based sample. The American Journal of Psychiatry, 172(12), 1215–1223. 10.1176/appi.ajp.2015.14101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnard, C. , Rane, L. J. , Wooderson, S. C. , Markopoulou, K. , Poon, L. , Fekadu, A. , … Cleare, A. J. (2014). The impact of childhood adversity on suicidality and clinical course in treatment‐resistant depression. Journal of Affective Disorders, 152‐154, 122–130. 10.1016/j.jad.2013.06.037 [DOI] [PubMed] [Google Scholar]
- Tyrka, A. R. , Wyche, M. C. , Kelly, M. M. , Price, L. H. , & Carpenter, L. L. (2009). Childhood maltreatment and adult personality disorder symptoms: Influence of maltreatment type. Psychiatry Research, 165(3), 281–287. 10.1016/j.psychres.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff, S. J. A. , Pannekoek, J. N. , Veer, I. M. , Van Tol, M. J. , Aleman, A. , Veltman, D. J. , … van der Wee, N. J. A. (2013). Resting‐state functional connectivity in adults with childhood emotional maltreatment. Psychological Medicine, 43(09), 1825–1836. [DOI] [PubMed] [Google Scholar]
- van Harmelen, A. L. , van Tol, M. J. , van der Wee, N. J. , Veltman, D. J. , Aleman, A. , Spinhoven, P. , … Elzinga, B. M. (2010). Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry, 68(9), 832–838. 10.1016/j.biopsych.2010.06.011 [DOI] [PubMed] [Google Scholar]
- Wacker, J. , Dillon, D. G. , & Pizzagalli, D. A. (2009). The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. NeuroImage, 46(1), 327–337. 10.1016/j.neuroimage.2009.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Liu, W. H. , Li, Z. , Wei, X. H. , Jiang, X. Q. , Geng, F. L. , … Chan, R. C. (2016). Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychological Medicine, 46(1), 125–135. 10.1017/S0033291715001592 [DOI] [PubMed] [Google Scholar]
- Wilcox, C. E. , Teshiba, T. M. , Merideth, F. , Ling, J. , & Mayer, A. R. (2011). Enhanced cue reactivity and fronto‐striatal functional connectivity in cocaine use disorders. Drug and Alcohol Dependence, 115(1–2), 137–144. 10.1016/j.drugalcdep.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G. , & Zang, Y. F. (2010). DPARSF: A MATLAB toolbox for "pipeline" data analysis of resting‐state fMRI. Frontiers in Systems Neuroscience, 4(13), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Helpman, L. , Papini, S. , Schneier, F. , Markowitz, J. C. , Van Meter, P. E. , … Neria, Y. (2017). Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depression and Anxiety, 34(7), 641–650. 10.1002/da.22594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings of the study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


