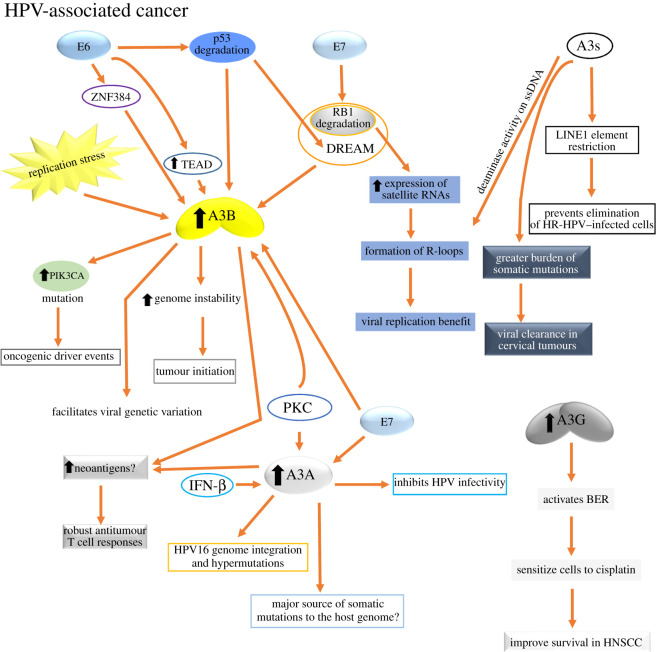
Figure 6.
Causes and effects of A3s upregulation in HPV-associated cancer. In these types of cancers, the downregulation of pRB and p53 tumour suppressors exerted by E7 and E6 oncoproteins, respectively, seems to be the main mechanisms by which the mutagenic activity of A3B and A3A is triggered. Other causes for A3B upregulation are replication stress and PKC signalling activation, with the latter being also a cause for A3A upregulation. The upregulation of A3B may benefit the virus by facilitating viral genetic variation or by helping to transform the cells. It was proposed that A3A, rather than A3B, may be the major source of somatic mutations to the host genome in HPV-associated cancer. Abundant neoantigens in these types of cancers may be associated with the deaminase activity of upregulated A3A and A3B expression that may be beneficial for tumour immunotherapy. A3A is also involved in the inhibition of the HPV infectivity, genome integration and hypermutation. The activity of A3s against retroelements could ameliorate the loss of LINE1 silencing caused by E7 inhibition of RB1. Also, HR-HPV-mediated RB1 degradation causes high-level expression of satellite RNAs that can lead to the formation of R-loops. A3s target the ssDNA in R-loops and can thereby also activate the DNA damage response, which benefits the virus replication. Cervical infections with a greater burden of somatic HPV16 A3-induced mutations are more likely to be benign or subsequently cleared. Lastly, higher A3G expression in HNSCC activates BER, sensitizing the tumour cells to cisplatin treatment and, consequently, improving the patient survival. For more details, see §5.4.