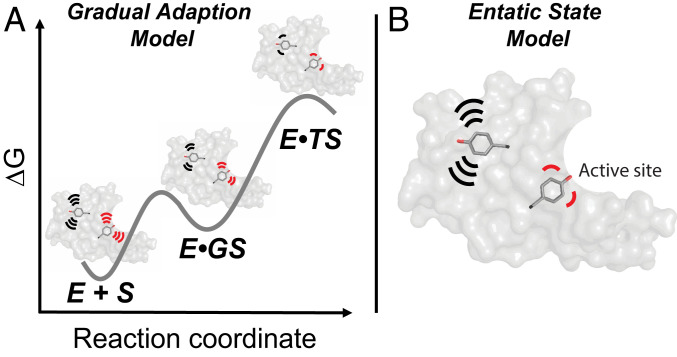
Fig. 9.
Models for conformational heterogeneity in enzyme catalysis. Each model is updated from original proposals, as described in the text, to incorporate an ensemble perspective (3, 5). Each panel shows an enzyme with two highlighted tyrosine residues, a “noncatalytic” tyrosine (black) in the enzyme core (gray) representing the noncatalytic residues, and a “catalytic” tyrosine (red) to represent catalytic residues in the active site. (A) The gradual adaption model (78). Both noncatalytic and catalytic tyrosine residues become more conformationally restricted as the reaction proceeds to the transition state. (B) The entatic state model (80, 81). Folding energy and local interactions provide greater restrictions and more precise positioning of the catalytic tyrosine (red, representing active site residues), relative to a noncatalytic tyrosine (black, representing noncatalytic residues). The restriction of active site residues (reduced conformational heterogeneity) is “paid for” with folding free energy and is used to enhance catalysis according to this model. In both panels, motions are schematically depicted by the motion lines.