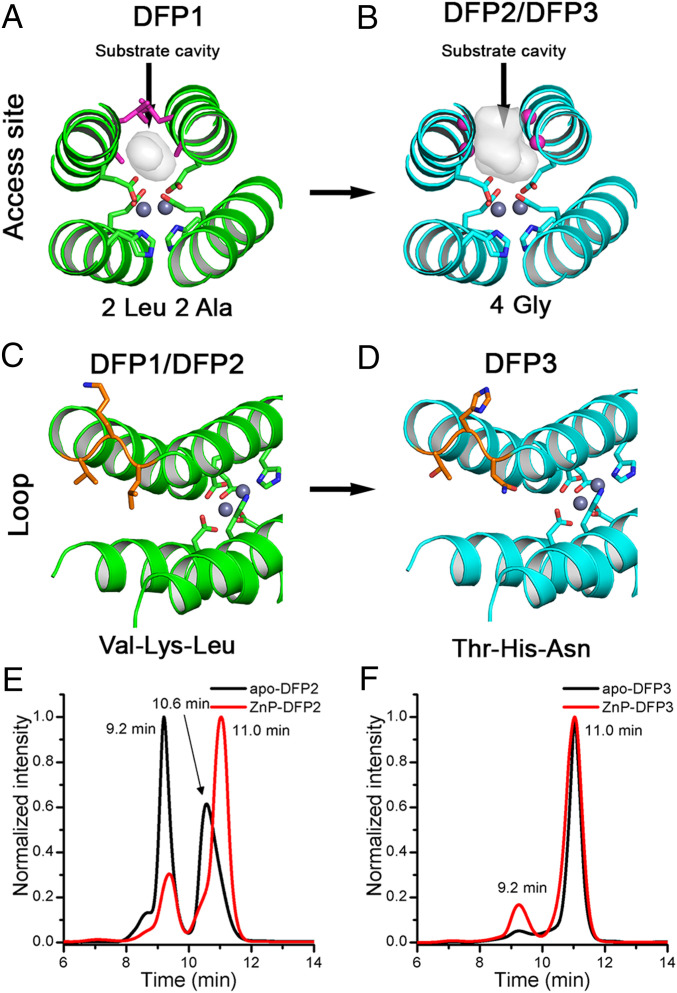
Fig. 3.
Engineering functionality in DFP family. The substrate cavity at the dimetal site was broadened mutating the 2 Leu and 2 Ala residues in DFP1 (shown as magenta sticks in A) in 4 Gly residues in DFP2 and DFP3 (shown as magenta spheres in B). To improve the oligomeric behavior, we mutated the Val-Lys-Leu loop in DFP1 and DFP2 (shown as orange sticks in C) in Thr-His-Asn in DFP3 (shown as orange sticks in D). The backbone is shown as green and cyan cartoon before and after the mutations, respectively. The coordinating residues of the dimetal center are shown as sticks, and the metal ions in gray spheres. (E) SEC of apo-DFP2 (in black) and ZnP-DFP2 (in red) at pH 7 (Hepes 50 mM, NaCl 100 mM), followed at 280 nm. (F) SEC of apo-DFP3 (in black) and ZnP-DFP3 (in red) at pH 7 (Hepes 50 mM, NaCl 100 mM), was followed at 280 nm. Retention time peaks at 10.6 min and 11.0 min corresponded to hydrodynamic radii of 27 Å and 21 Å, respectively.