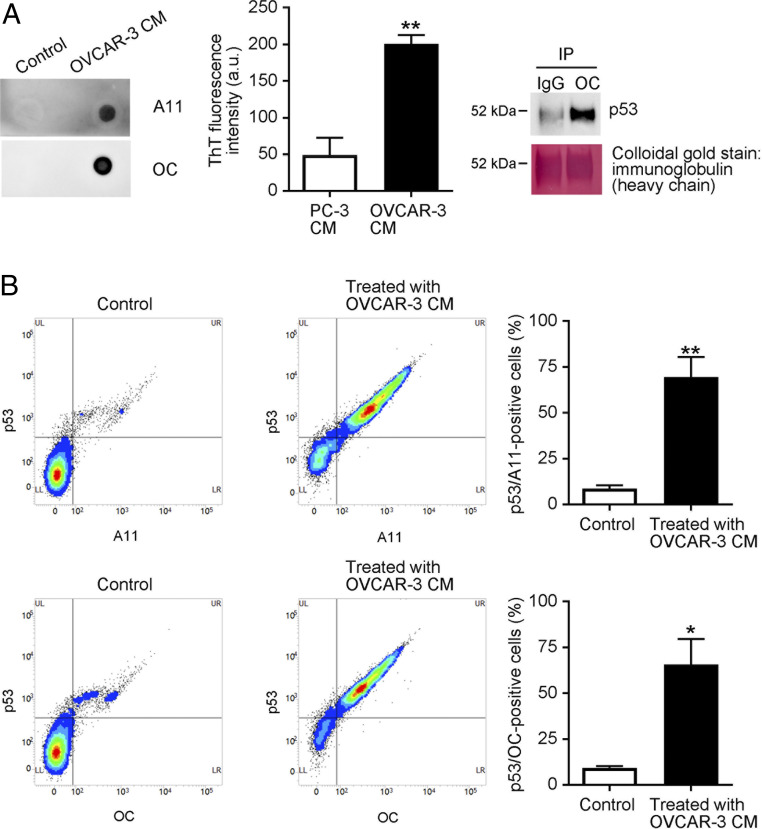
Fig. 2.
CHO cells take up cancer cell-derived p53 aggregates. (A) Human ovarian cancer-derived OVCAR-3 cells released p53 aggregates. OVCAR-3 cells were cultured in serum-free OPTI-MEM for 12 h, after which samples of culture media were collected (OVCAR-3 CM). Protein aggregates in the media were analyzed by means of dot blotting with the A11 anti-oligomer or the OC anti-amyloid fibril antibody (“Control” indicates that fresh RPMI was spotted). ThT fluorescence intensity (10 µM) in CM of PC-3 cells (p53-null cells) and OVCAR-3 cells was recorded at 485 nm with an excitation wavelength of 445 nm (Center) by a multigrating high-speed microplate reader. Amyloid fibrils were immunoprecipitated with the OC anti-amyloid fibril antibody, and the immunoprecipitates were then subjected to Western blotting with the DO-1 anti-p53 monoclonal antibody, which showed that the OC-reactive amyloid fibrils consisted of p53. After examination, the membrane was stained with colloidal gold. (B) CHO WT cells were treated with OVCAR-3 CM for 8 h, after which cells were collected and analyzed by means of FACS with the DO-1 anti-p53 antibody and the A11 and OC anti-protein aggregate antibodies. Detection of cells positive for both p53 and protein aggregates confirmed that CHO cells successfully took up OVCAR-3-derived p53 aggregates. “Control” means that CHO cells were treated with fresh RPMI. The graph shows quantification of p53-positive and A11-positive or OC-positive cells. Data are means ± SEM of three independent experiments. *P < 0.05; **P < 0.01.