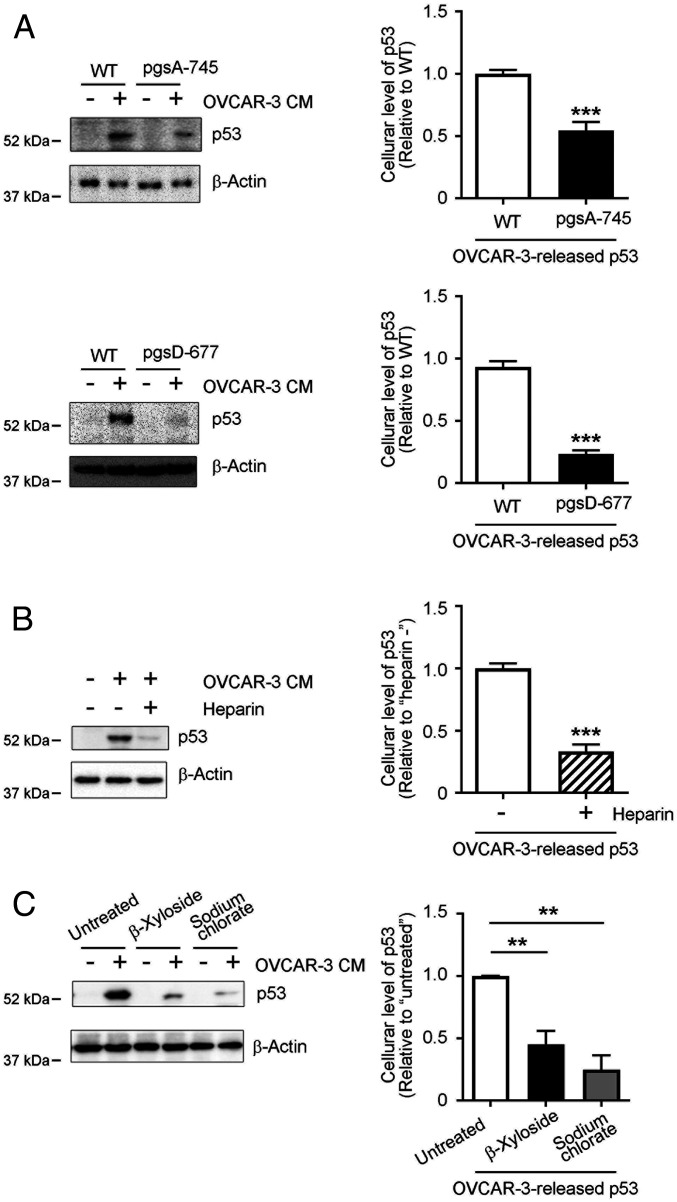
Fig. 3.
Cellular uptake of OVCAR-3 cell-derived p53 aggregates depends on sulfated GAGs at the cell surface. (A) Significantly reduced uptake of cancer cell-derived p53 by pgsA-745 and pgsD-677 CHO mutant cells. WT, sulfated GAG-deficient pgsA-745, and HS-deficient/CS-sufficient pgsD-677 CHO cells were treated with OVCAR-3 CM for 8 h, after which levels of p53 proteins in whole cell lysates were analyzed by means of Western blotting. (B) Exogenously added heparin, an HS S-domain analog, interfered with cellular uptake of cancer-cell derived p53. CHO cells were treated with OVCAR-3 CM with or without heparin (5 μg/mL), after which whole-cell lysates were prepared and p53 protein levels in the lysates were determined by means of Western blotting. (C) Removal of cell surface sulfated GAGs or inhibition of cellular sulfation modifications significantly inhibited cellular uptake of cancer cell-derived p53. CHO WT cells were pretreated with 2.5 mM β-xyloside or 100 mM sodium chlorate in OPTI-MEM for 24 h, after which cells were treated with OVCAR-3 CM for 8 h. The presence of p53 proteins in whole-cell lysates was determined by means of Western blotting. β-Actin was used as a loading control. Graphs show quantification of cellular uptake of cancer cell-derived p53 aggregates. Data are means ± SEM of three independent experiments. **P < 0.01; ***P < 0.001.