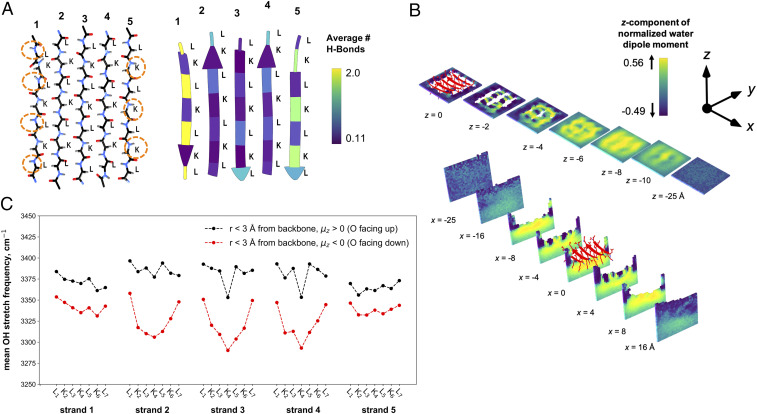
Fig. 4.
Hydrogen-bond and water structure analyses of the (l-) LK7β homopentamer at the vacuum–water interface obtained from 1 μs of MD sampling using the AMBER ff14SB force field for the protein and the TIP4P-Ew water potential. (A, Left) LK7β backbone atoms show the asymmetry of the N-H and C = O groups on opposite sides of the β-sheet. (A, Right) The average number of hydrogen-bonding interactions between the protein and water molecules depicted by residue. (B) The average z-components of water dipole moment vectors extending below the protein surface (Top) and from side to side (Bottom) with a grid resolution of 1 Å. (C) The infrared response of O-H stretch of water within 3 Å of the peptide backbone for every residue. The IR response was calculated for water molecules where the average z-component of the dipole was oriented either up (black) or down (red) relative to the vacuum–water interface.