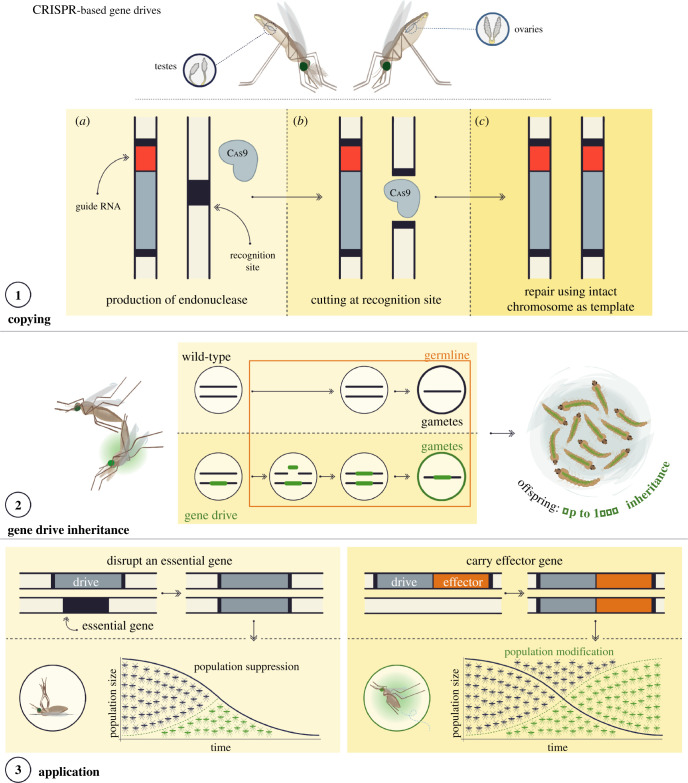
Figure 1.
Mode of inheritance of CRISPR-based gene drives and their potential applications. Panel 1: the gene drive cassette consists of a source of Cas9 under a germline-specific promoter and a guide RNA designed to target the Cas9 to recognize and cut the target site on a wild-type chromosome. Because the gene drive is initially inserted within its own recognition site the chromosome containing the gene drive is immune from cleavage. Thus in an individual heterozygous for the gene drive, the wild-type chromosome is cut. The homology-directed repair pathway, invoked by the host cell, causes the broken ends of the cleaved chromosome to invade the intact homologous chromosome and pair at regions of homology before then using templated DNA synthesis along the intervening sequence, followed by resolution of the two chromosomes once this is complete. This ‘homing' process leads to a duplication of the gene drive so that it is now on both chromosomes. Panel 2: if the homing process happens in the germline prior to gamete formation then a higher proportion of the gametes from heterozygous individual will contain a copy of the gene drive than the 50% that would be expected if normal Mendelian inheritance applied. In Anopheles mosquitoes, the homing process can be very efficient and in some cases the rate of inheritance of a synthetic homing gene drive can approach 100%. Homing can occur in the germline of both sexes. Panel 3: left panel: population-wide knockout of some essential gene that produces a desirable phenotype (from a malaria control perspective) in individuals homozygous for the gene drive, such as female infertility, by virtue of the gene drive disrupting the coding sequence of the target gene. Importantly for this approach to work most effectively, ‘carrier' individuals heterozygous for the drive should be fully viable. right panel: population-wide spread of an effector gene as ‘cargo’. Gene drives that interfere with mosquito reproduction or viability aim to the eliminate or suppress the population to levels that do not support disease transmission (left panel). Alternatively, gene drives engineered to directly impair vector competence can invade the vector population, transforming it over time to become one that is refractory to malaria transmission (right panel).