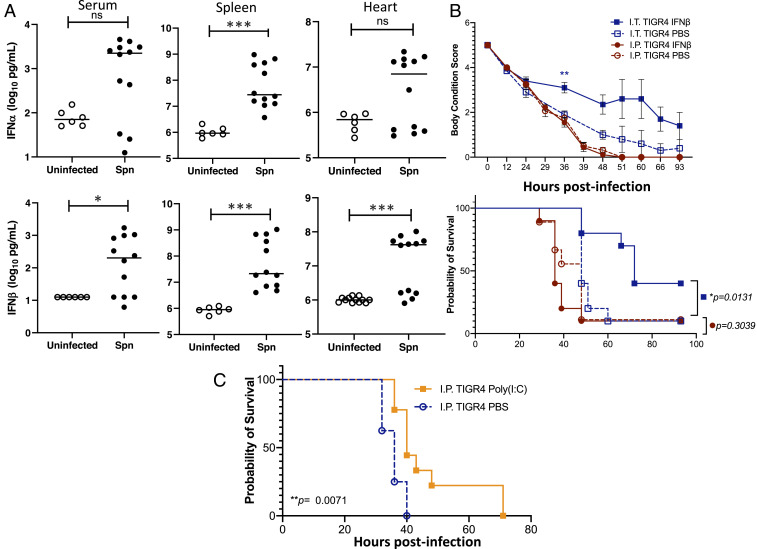
Fig. 7.
Assessment of the IFN response and its contribution to mouse protection. (A) IFN-α and IFN-β levels in the serum, spleen, and hearts of mice experiencing invasive pneumococcal disease. Each data point represents an individual mouse with statistical analysis performed using a two-tailed Student’s t test (*P < 0.05; ***P < 0.001). (B) Mice were administered recombinant IFN-β (30,000 U) in saline or saline alone either intranasally (I.N.; 50 µL volume) or intraperitoneally (I.P.; 100 µL volume) 24 h prior to challenge intratracheally (I.T.) with 5 × 106 CFU or intraperitoneally with 1 × 105 CFU of TIGR4 in 100 µL of saline, respectively. Body condition score and Kaplan–Meier survival of mice treated with IFN-β versus control following challenge with TIGR4. Body condition score significance was tested using a repeated-measures mixed-effects model with Sidak’s multiple comparison posttest of IFN-β versus saline only within each infection group (**P < 0.01); and survival significance was assessed by log-rank Mantel–Cox test (P values indicated on the graph; n = 10 mice per treatment group). (C) Mice were administered 50 µg Poly(I:C) in 100 µL saline intraperitoneally 24 h prior to challenge, intraperitoneally with 1 × 105 CFU of TIGR4. Survival significance was assessed by log-rank Mantel–Cox test (P value indicated on the graph; n = 9 mice per treatment group).