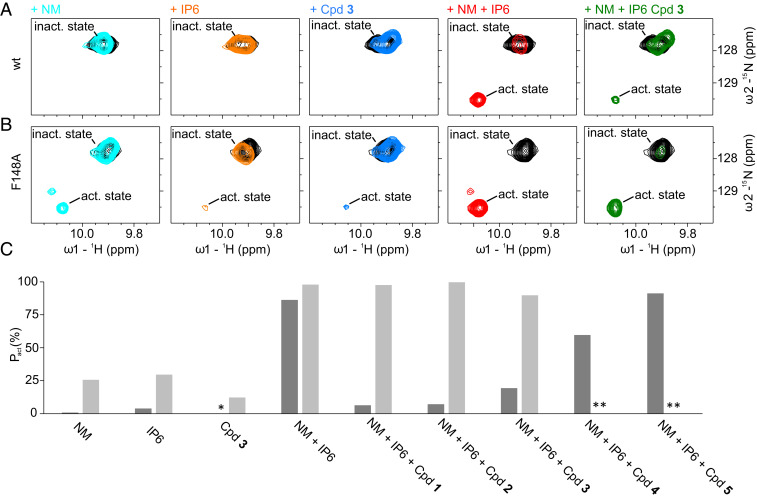
Fig. 3.
(A and B) The region of the NMR 1H,15N-correlation spectra of the MLKL executioner domain (protein concentration: 80 µM) containing the cross-peaks of Trp133 Nε1–Hε1 in the auto-inhibited state where α-helix 6 is attached to the four-helix bundle (inact. state) and in the active, detached state (act. state) are shown. The unliganded state is depicted in black, with 12 mM NM in cyan; 500 µM IP6 in orange; 500 µM Cpd 3 in blue; 12 mM NM and 500 µM IP6 in red; and 12 mM NM, 500 µM IP6, and 500 µM Cpd 3 in green. The spectra depicted in A are measured with the WT protein and in B with the F148A variant. (C) Population of the active conformation (pact) in percent where α-helix 6 is detached from the four-helix bundle calculated from integrals of Trp133 Nε1–Hε1 cross-peaks in the active and inactive states. Dark gray bars correspond to the WT, while light gray bars correspond to the F148A variant. The asterisk means that no active conformation could be detected. Double asterisks indicate data not determined.