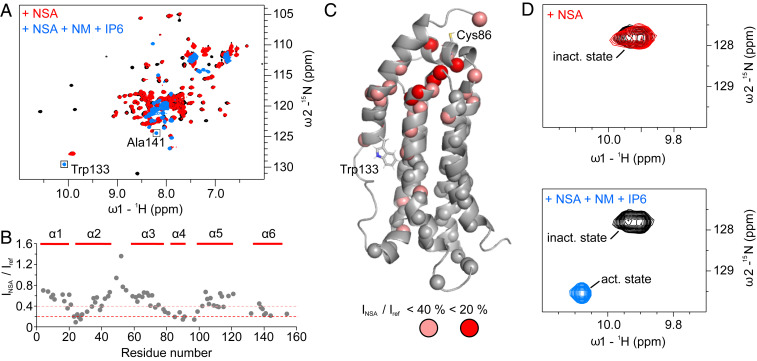
Fig. 6.
(A) NMR 1H,15N-correlation spectrum of 80 µM unliganded MLKL executioner domain (black), in complex with NSA (red) at a concentration of 200 µM, which is sufficient to saturate the protein, and additionally in the presence of 12 mM NM and 500 µM IP6 (blue). (B) Ratio of the peak intensities of the MLKL executioner domain:NSA complex over the unliganded protein extracted from spectra shown in A. (C) NMR structure of the MLKL executioner domain. Spheres indicate residues for which the peak height in the unliganded and the NSA bound state could be compared. Salmon spheres indicate residues that have peak intensities smaller than 40%, and red spheres residues with peak intensities smaller than 20% in the NSA bound state. (D) Regions of the spectrum the 1H,15N-correlation spectra containing the Trp133 Nε1–Hε1 cross-peaks in the auto-inhibited state where α-helix 6 is attached to the four-helix bundle (inact. state) and in the active state where it is detached (act. state).