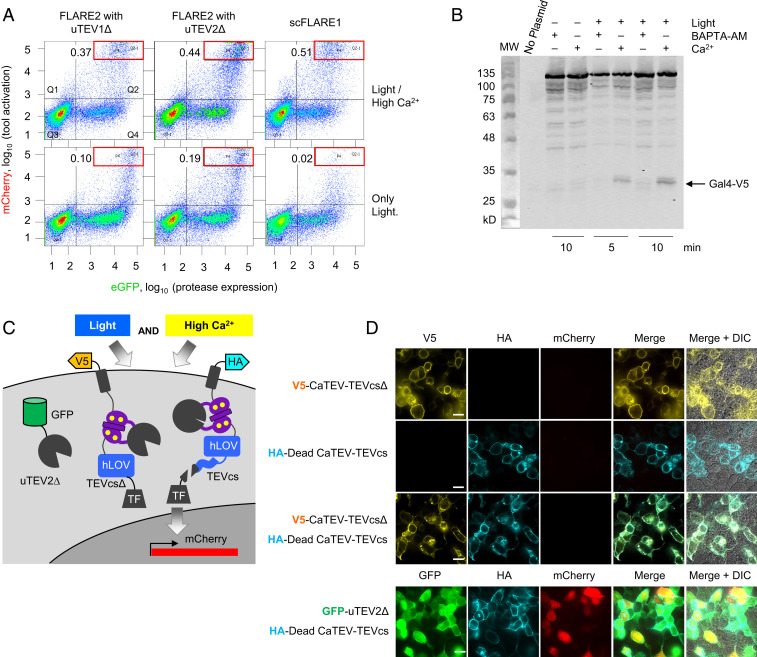
Fig. 2.
Characterization of scFLARE1 in HEK 293T cells. (A) scFLARE1 has lower background than two-component FLARE2 (16). HEK 293T cells expressing the indicated constructs and UAS:mCherry were stimulated for 5 min and analyzed by FACS 8 h later. GFP is fused to TEV protease in FLARE2, and it is expressed via p2a in scFLARE1 (TM-CaTEV(uTEV1Δ)-hLOV-TEVcs-Gal4-p2A-GFP). Ratios in the top left of each FACS plot indicate the number of cells within the red box region divided by the number of cells in Q4. (B) Western blot analysis of scFLARE1 cleavage. HEK cells expressing scFLARE1 were prepared and stimulated (for 5 or 10 min) as in Fig. 1E. Immediately after stimulation, cells were lysed in the presence of 2 mM iodoacetamide (TEV protease inhibitor) and run on 9% SDS/PAGE. scFLARE1 is 145 kDa before cleavage and 32 kDa (V5-containing portion) + 113 kDa after cleavage. BAPTA-AM was added to control cells not receiving Ca2+ stimulation. (C) Constructs used to test scFLARE1 cleavage mechanism. The V5-tagged TF construct lacks a TEVcs. The HA-tagged TF has an inactive protease (C151A mutation (31); DeadCaTEV). (D) The indicated constructs were expressed in HEK 293T cells along with UAS:mCherry. Cells were stimulated for 5 min with light and Ca2+ and then fixed, stained, and imaged 8 h later. The bottom row coexpresses high-affinity TEV protease (GFP tagged) with the HA-tagged DeadCaTEV construct to show that the latter can still be cleaved to generate mCherry reporter signal. Scale bars, 10 μm.