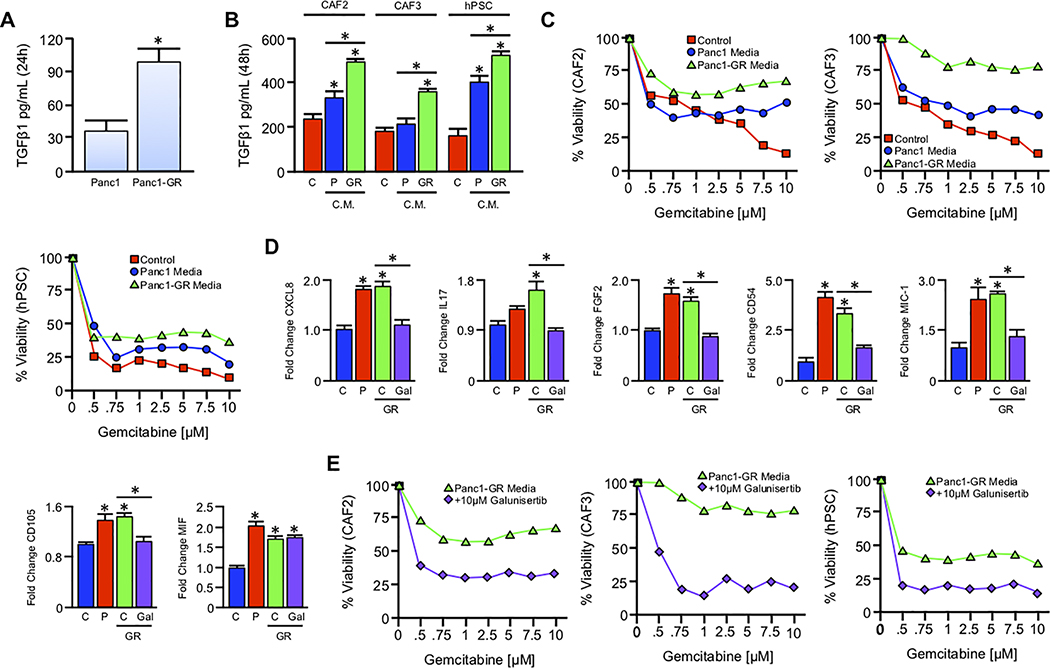
Figure 4. Gemcitabine-resistant tumor cells confer drug resistance to stromal cells via paracrine TGFβ signaling.
(A) An equal number of Panc1 cells and Panc1-GR cells were seeded into 6-well plates and grown in serum free media. After 24 hours, culture media was collected, filtered, and subjected to TGFβ1 ELISA (*p < 0.05). (B) Serum free media was conditioned in either Panc1 or Panc1-GR cells for 24 hours as described and sterile filtered. Media was supplemented with 10% FBS, and transferred to an equal number of CAF2, CAF3, or hPSC stromal cell lines. After 48 hours, control (C), Panc1 conditioned (P), and Panc1-GR (GR) conditioned media was collected and re-evaluated for TGFβ by ELISA (*p < 0.05). (C) CAF2, CAF3, and hPSC cells were incubated with increasing concentrations of Gemcitabine delivered in either control media (red), Panc1 conditioned media (blue), or Panc1-GR conditioned media (GR, green). After 72 hours cell viability was evaluated by MTT assay. (D) hPSC cells were grown in either control (C), Panc1 conditioned (P), Panc1-GR (GR) conditioned media, or GR media with 10μM of the TGFBR1-inhbitor Galunisertib. After 48 hours, cells were incubated with a protein transport inhibitor for one hour, lysed, and 200μg of total cell lysate was evaluated by a high throughput proteome profiler array (ARY022B). Pixel density was evaluated using ImageJ, and samples normalized to the mean intensity of the reference spots for each blot minus the background density. Mean normalized values for experimental groups were divided by those for untreated hPSC cells, and are presented as fold change plus standard deviation (*p < 0.05). (E) CAF2, CAF3, and hPSC cells were again incubated with increasing concentrations of Gemcitabine delivered in either Panc1-GR conditioned media (green) or Panc1-GR conditioned media supplemented with 10μM of the TGFBR1-inhbitor Galunisertib (purple). After 72 hours cell viability was evaluated by MTT assay.