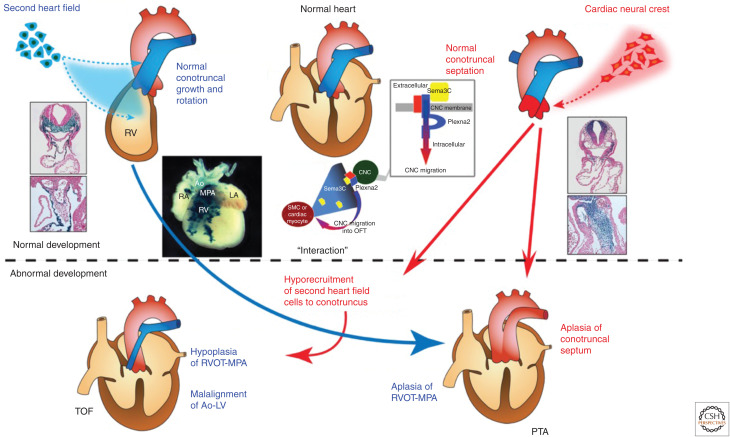
Figure 4.
Normal and abnormal development of the cardiac neural crest (CNC) and the second heart field (SHF) and their interaction implicated in the spectrum of outflow tract (OFT) defects. The SHF gives rise to the OFT myocardium and CNC gives rise to the OFT septum, respectively. Although the precise embryological mechanism remains uncertain, tetralogy of Fallot (TOF) is believed to result from malrotation of the OFT that leads to misalignment of the outlet and trabecular septum, and consequent overriding of the aorta (Ao) above the malaligned ventricular septum. Contribution of CNC is thought to be essential for proper rotation and septation of the OFT. Alternatively, hypoplasia and underdevelopment of the pulmonary infundibulum may also be responsible for the infundibular obstruction and malalignment of the outlet septum. Cre-mediated transgenic system in mice revealed that a subset of cells derived from the SHF contribute predominantly to the pulmonary infundibulum. Accordingly, developmental defects of the SHF may cause hypoplasia of the pulmonary infundibulum, resulting in TOF, and more severe decreased number or absence of this subset of cells may affect development and/or migration of CNC, resulting in persistent truncus arteriosus (PTA). This notion is consistent with the observation that OFT defects ranging from TOF to PTA are highly associated with 22q11DS. Recent studies suggested that reciprocal molecular signaling between SHF and CNC, such as semaphorin 3C (Sema3C) ligand expressed in the SHF and plexin A2 (Plexna2) and neuropillin-1 (Npn-1) receptors expressed in the CNC, was essential for correct navigation of CNC cells toward the SHF-derived OFT, eventually resulting in proper alignment and septation of the OFT. (RV) right ventricle, (RA) right atrium, (LA) left atrium, (MPA) main pulmonary artery, (RVOT) right ventricular outflow, (SMC) smooth muscle cell.