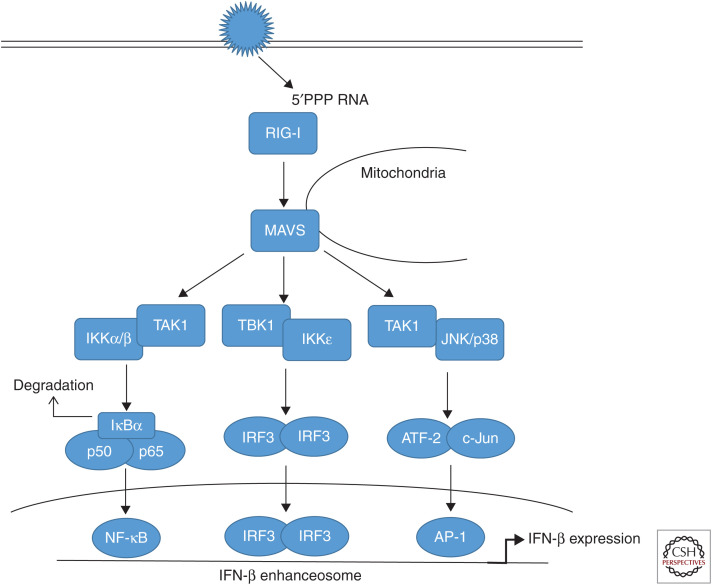
Figure 1.
Retinoic acid inducible gene (RIG)-I-dependent type I interferon induction. During infection of host cells with influenza viruses (IVs), incoming or newly synthesized 5′-triphosphorylated viral RNA (5′PPP RNA) is sensed as a pathogen-associated molecular pattern (PAMP) by the ubiquitously expressed cytoplasmic sensor RIG-I. RIG-I triggers via the adapter MAVS (mitochondrial antiviral signaling) signal transduction cascades that result in the activation of different transcription factors. The major MAVS signaling complex consist of tumor necrosis factor (TNF) receptor–associated factor (TRAF)3 and TANK-binding kinase 1 (TBK1) inducing inhibitor of IĸB-kinase-ε (IKKε) activity. This results in the phosphorylation of the constitutively expressed interferon (IFN) regulatory factor 3 (IRF3) as well as the induced factor IRF7, belonging to the set of IFN-stimulated genes (ISGs) induced during infection. IRF3 and IRF7 homodimers as well as IRF3/7 heterodimers translocate into the nucleus to induce synthesis of type I IFN mRNAs via binding to the IFN-β enhanceosome. A second signaling complex consisting of TRAF6 and TGF-β-activated kinase 1 (TAK1) induces the activation of IKKβ. IKKβ phosphorylates the inhibitor of ĸB (IκBα), a protein masking the nuclear localization signals (NLSs) of nuclear factor (NF)-κB transcription factors, keeping them sequestered in an inactive state in the cytoplasm. This phosphorylation results in the dissociation of IκBα from NF-κB, which migrates into the nucleus. Additionally, TAK1 activates mitogen-activated protein kinases (MAPKs) p38 and JNK (c-Jun amino-terminal kinase) resulting in the phosphorylation of activating transcription factor 2 (ATF-2) and c-Jun, which dimerize and translocate to the nucleus. Activity of these transcription factors collectively mediates full activation of the IFN-β promoter.