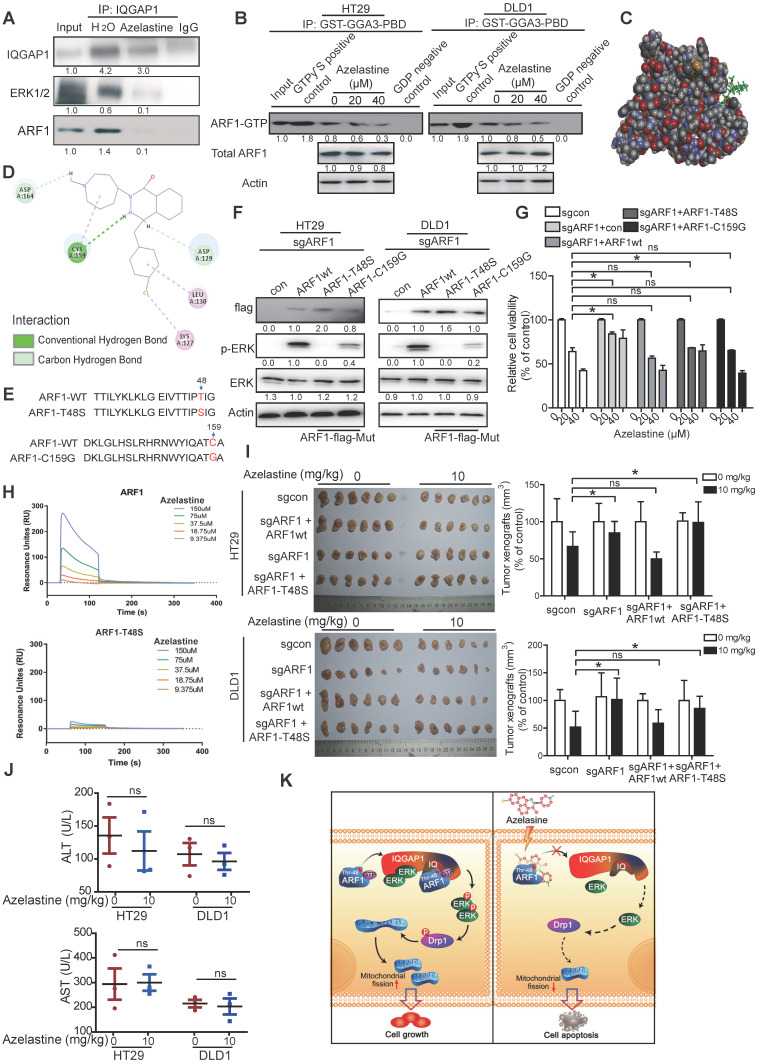
Figure 6.
Thr-48 of ARF1 is required for the anticancer bioactivity of azelastine. (A) Immunoprecipitation assay showing that the IQGAP1 interactions with ARF1 and ERK were blocked by azelastine. (B) ARF1 activation assay was used to measure ARF1 activity in HT29 and DLD1 cells with various concentrations of azelastine treatment for 48 h. (C) Molecular docking was used to analyze the potential binding site involved in the combination of azelastine and ARF1. (D) 2D diagram showing the bonding of the drug azelastine and the protein ARF1. (E) Mutation design of ARF1-T48S and ARF1-C159G. (F-G) Wild-type ARF1, ARF1-T48S mutant, or ARF1-C159G mutant was overexpressed in ARF1-deficient HT29 and DLD1 cells, and p-ERK expression was analyzed (F), and the WST-1 assay was performed to compare the sensitivity of CRC cell lines to azelastine treatment (G). (H) Biacore results showed that ARF1-T48S mutant protein had significantly weaker interaction with azelastine than wild-type ARF1. (I) ARF1-deficient HT29 and DLD1 cells were overexpressing wild-type ARF1 or ARF1-T48S mutantwere used to establish tumor xenografts. The nude mice were orally administrated with azelastine (10 mg/kg) or vehicle every two days. Note that azelastine could not inhibit the ability of ARF1-deficient CRC cells to form tumors, and the antitumor effect of azelastine was recovered when the cells were re-overexpressed with wild-type ARF1, but not with mutant ARF1. (J) ALT) and AST in mice serum. (K) Schematic diagram illustrating that azelastine binds to ARF1 to inhibit mitochondrial fission and suppress colon tumorigenesis. Bars, SD; *, P < 0.05; **, P < 0.01; ***, P < 0.001.