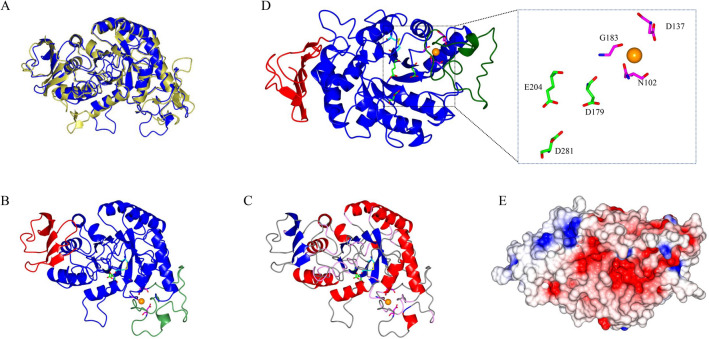
Fig. 3.
The homology structural model of the M. timonae CTI-57 AMY1 α-amylase. a A superposition of the modeled protein, in blue, with the used template (PDB 1AMY), in gold. b Domains A, B, and C are shown in blue, green, and red, respectively. The predicted disulfide bond formed between Cys201-Cys232 is shown in yellow with the Cys residues in cyan. The Ca2+ ion is displayed in orange. c The α-helices, β-strands, turns, and coils are shown in red, blue, pink, and gray, respectively. The A domain, constituted by the (β/α)8-barrel, can be seen in the center. The domain B protrudes between β3-strand and α3-helix of domain A. The C-terminal domain is constituted of five antiparallel β-strands. d The catalytic residues are shown in green and red. The Ca2+ coordinating residues are shown in magenta and red. e Electrostatic potential (red—negative regions; blue—positive regions) in the protein surface positioned as in C)