Table 5.
Ultrasound assisted synthesis of compound 3c-j.a
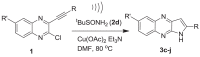 | |||
| 1; R’, R = | Time | Product (3) | %Yieldb |
| 1a; H, Ph | 1.5 | 3c | 67 |
| 1b; H, C6H4Me-p | 1.5 | 3d | 71 |
| 1c; H, t-Bu | 2 | 3e | 59 |
| 1d; H, CMe2OH | 1.5 | 3f | 63 |
| 1e; H, n-Bu | 1.5 | 3g | 73 |
| 1f; H, H | 2 | 3h | 60 |
| 1g; Me. Ph | 4 | 3i | 65 |
| 1h; Me, C6H4Me-p | 1.5 | 3j | 62 |
All the reactions were carried out by using 1 (2.5 mmol), 2d (2.8 mmol), Cu(OAc)2 (0.025 mmol) and Et3N (3.77 mmol) in DMF (3 mL) under ultrasound.
Isolated yield.
