ABSTRACT
Background
Restaurant oil in poultry diets increases energy content, reduces production costs, and promotes sustainability within the food supply chain. However, variable oil composition and heating temperatures among restaurant oil sources can impact broiler chicken health due to heat-induced lipid modifications.
Objectives
A 21-d experiment was conducted to evaluate ileal morphology, liver cytokine gene expression, and ileal immune cell populations in broilers fed control or peroxidized lipids with varying chain and saturation characteristics.
Methods
Day-old broilers were housed in battery cages (5 birds per cage) and fed diets containing 5% control or peroxidized oils. Eight diets were randomly assigned in a 4 × 2 factorial arrangement consisting of oil source (palm, soybean, flaxseed, or fish) and peroxidation status (control or peroxidized). At day 21, samples were collected for ileal histomorphology [villus height (VH), crypt depth (CrD), and the VH:CrD ratio], and liver cytokine expression (qPCR). Ileum cytokine expression and T-cell markers were analyzed by RNAscope in situ hybridization (ISH). Data were analyzed as a mixed model (SAS 9.4) with fixed effects of lipid source, peroxidation, and lipid × peroxidation interaction.
Results
CD3+ T-cells in the ileum decreased 16.2% due to peroxidation (P = 0.001) with 30.3% reductions observed in birds fed peroxidized flaxseed oil (P = 0.01). Peroxidation increased IL6+ and IL1B+ cells by 62.0% and 40.3%, respectively (P = 0.01). Soybean oil increased IFNG+ cells by 55.1% compared with palm oil, regardless of peroxidation status (P = 0.007). Lipid source and peroxidation did not alter ileal histomorphology or liver cytokine expression.
Conclusions
Lipid peroxidation increased ileal IL1B and IL6 in broiler chickens, whereas soybean oil diets increased IFNG. Generally, peroxidation decreased overall CD3+ T-cell populations, suggesting impaired T-cell presence or recruitment. These results identify potential immunomodulatory lipid profiles in restaurant oil while supporting RNAscope-ISH as a method to describe avian tissue-level immune responses.
Keywords: broilers, immunity, peroxidized oils, RNAscope, ileum
Introduction
Immune responses to dietary oils vary based on fatty acid (FA) composition, particularly with the degree of saturation and double bond location. Lipid sources high in SFAs are linked to underlying inflammatory states related to obesity, whereas the inflammatory potential of unsaturated fatty acids (UFAs) is related to structural properties (1, 2). Dietary omega-3 FAs have been implicated in a variety of beneficial anti-inflammatory and immunomodulatory mechanisms in human and animal models at varying health states (3–8). Oils high in n–6 PUFAs are regarded as proinflammatory due to their conversion to arachidonic acid and subsequent production of proinflammatory mediators (9, 10). In contrast, oils containing a high concentration of n–3 PUFAs are regarded as anti-inflammatory due to their antagonistic role in arachidonic acid production and inhibition of proinflammatory cytokine gene expression, but can exhibit varying degrees of bioreactivity (11). Although the inflammatory designations for these FAs are generally accepted, conflicting reports of the pro- and anti-inflammatory properties of n–6 and n–3 FAs have been published (12–14).
Processes that modify lipid structure, such as heating, have been implicated in changing functional growth and immune responses in several animal models (15–18). Restaurant oils (i.e., yellow grease) are subjected to prolonged and repeated exposure to high temperatures, which leads to the formation of potentially harmful peroxidation products like hydroperoxides and aldehydes. Although use of these oils in animal agriculture provides an economical alternative energy source for producers (19–21), yellow grease with high concentrations of free fatty acids and lipid peroxidation products has been shown to negatively impact broiler performance (22). Additionally, other groups indicated that feeding peroxidized corn oil decreased nitrogen-corrected apparent metabolizable energy but had little impact on bird performance (23). The work herein describes the immunological outcomes observed as part of a larger study that additionally examined and published performance and oxidative stress responses to lipid source and peroxidation. From this we previously reported that feeding peroxidized lipid sources reduced performance and serum glutathione peroxidase activity while increasing liver carbonyls in broilers (24). Likewise, the detrimental effects of feeding peroxidized lipid sources on average daily gain and measures of oxidative stress, such as elevated serum TBARS and reduced liver glutathione peroxidase activity, have also been observed in swine (15, 25–27).
Oil sources high in PUFAs are more susceptible to peroxidation and structural changes caused by heat exposure, which have the potential to elicit an immune response (17, 28–31). Lymphocytes harvested from chicks fed fresh, unmodified flaxseed and fish oils showed decreased proliferation in vitro (32), whereas diets containing 0.5–2.5% fish oil decreased the acute-phase response and proinflammatory IL-1 in LPS-challenged chicks (33, 34). Similarly, increasing inclusion of n–3 PUFAs in broiler diets elevated serum concentrations of IL-2 and IFN-γ in addition to decreased phagocytosis and lymphocyte proliferation in cells harvested from unchallenged broilers (35, 36). These findings suggest an anti-inflammatory impact of feeding n–3 PUFAs, but the comparative effect of different lipid sources on poultry immunity is not widely reported. The effects of lipid source and oil peroxidation on broad measurements such as performance have been described in poultry; however, underlying changes to intestinal morphology and immunity that could contribute to alterations in downstream performance are not as well defined. This is particularly important in the context of using yellow grease in broiler diets because this product comes from multiple sources, each with variable lipid composition and degrees of peroxidation.
Methods for studying the immune responses in poultry largely focus on cytokine responses, with qPCR being a commonly utilized method for studying cytokine expression in poultry tissues. This method is suitable for determining levels of tissue cytokine gene expression but lacks the ability to describe the immune response in detail. Here, to the best of our knowledge, we are the first to employ RNAscope in situ hybridization (ISH) as a method of detecting cytokine production and immune cell presence in the gastrointestinal tract of poultry. RNAscope utilizes different markers within the same tissue to provide simultaneous information about cytokine production and underlying immune cell populations. Previously, this method has been used to measure the production of antimicrobial peptides in the broiler intestine during an Eimeria challenge (37).
The selection of cytokines for analysis by qPCR and RNAscope was based on their role in the immune response. Cytokine genes IL1B, IL6, IL10, IL18, and IFNG were selected for qPCR analysis based on their function in the immune response, whereas PPARG (peroxisome proliferator–activated receptor γ) was selected based on its role as a regulator of adipogenesis (Supplemental Table 1; 38). Of these cytokines, IL1B, IL6, IL10, IL4, and IFNG were selected for RNAscope analysis. IL-1β and IL-6 are proinflammatory and function in the innate immune response and the transition between innate and adaptive responses, respectively (39, 40). In contrast, IL-10 is anti-inflammatory and functions to control the extent of an inflammatory response (41, 42). IFN-γ activates macrophages and natural killer cells while also functioning in both the innate and adaptive immune response (43, 44). CD3 and CD4 expression was visualized to determine ileal T-cell presence response to potential lipid antigens.
The oil sources in this study were selected based on factors such as chain length, saturation, and peroxidation susceptibility. Palm oil has a low ratio of UFAs to SFAs and as a result is less susceptible to the formation of peroxidation products. In contrast, soybean, flaxseed, and fish oils have higher UFA:SFA ratios and are more susceptible to peroxidation product formation with heat and air exposure. Within the UFAs used, soybean oil is rich in n–6 FAs such as linoleic acid whereas flaxseed and fish oils are enriched in n–3 FAs (45, 46). Although both are well-studied sources of n–3 FAs, the α-linolenic acid in flaxseed oil is a precursor to, and therefore less bioactive than, the EPA and DHA present in fish oil (47). This study was conducted to identify the effects of feeding these different lipid sources with varying peroxidation statuses on ileal histomorphology, liver cytokine gene expression, and lymphocyte populations among other cytokine-producing cell populations in the broiler chicken ileum.
Methods
All procedures involving animals were approved by the Iowa State University Institutional Animal Care and Use Committee.
Animal experiment overview
Two hundred 1-d-old broiler chicks were housed in raised wire battery cages (5 birds per cage) in an environmentally controlled room. All chicks received a starter diet for 3 d for acclimation to the battery pens and mash feed. On day 4, chicks were individually wing-banded, weighed, and randomly assigned to 1 of 8 experimental diets (experimental day 0). Battery cage was the experimental unit and there were 5 replicates per treatment. Broilers had ad libitum access to experimental diets and water for the duration of the experiment.
Dietary treatments were formulated based on genetic company recommendation to meet or exceed NRC recommendations (48) and were arranged in a 4 × 2 factorial within a completely randomized design (Table 1). Factors consisted of 4 oil sources: a 5% inclusion of palm oil, soybean oil, flaxseed oil, or fish oil; in combination with peroxidation status: control or peroxidized oil. Peroxidation was achieved by thermally processing the oils at 90°C for 72 h with a continuous infusion of air (3 L/min), whereas control oils had no thermal or air infusion treatment. Characterization (FA profile, oil quality, lipid peroxidation products, and total tocopherols) of each fresh or peroxidized oil, and the composition of diets has been reported previously (24) and select values are summarized in Table 2. On day 21, two broilers per pen were killed by carbon dioxide asphyxiation for tissue collection.
TABLE 1.
Composition of common basal diet fed to broiler chickens for the 20-d experimental period, as fed basis1
| Ingredient, g/kg | |
| Corn | 470.6 |
| Sybean meal | 434.1 |
| Oil2 | 50.0 |
| Dicalcium phosphate | 18.3 |
| Limestone | 11.0 |
| Vitamin/mineral premix3 | 6.3 |
| Sodium chloride | 5.0 |
| Methionine hydroxy analog | 3.4 |
| Choline chloride 60 | 1.0 |
| l-Threonine | 0.3 |
| Calculated composition, g/kg | |
| Crude protein | 251.0 |
| Crude fat | 73.0 |
| Dietary fiber | 26.1 |
| ME, kcal/kg | 3138 |
Adapted from compositions reported by Lindblom et al. (24). ME, metabolizable energy.
Oils added to the diet consist of control or peroxidized palm, soybean, flaxseed, or fish oil. Peroxidation was achieved by heating oils (90°C) for 72 h with constant air infusion (3 L/min).
Vitamin and mineral premix provided per kilogram of diet: selenium 250 μg; vitamin A (retinyl acetate) 2838 μg; cholecalciferol (vitamin D-3) 68.8 μg; α-tocopherol acetate (vitamin E) 13.2 mg; menadione 1.1 mg; vitamin B-12 12 μg; biotin 41 μg; choline 447 mg; folic acid 1.4 mg; niacin 41.3 mg; pantothenic acid 11 mg; pyridoxine 1.1 mg; riboflavin 5.5 mg; thiamine 1.4 mg; iron 282 mg; magnesium 125 mg; manganese 275 mg; zinc 275 mg; copper 27.5 mg; iodine 844 μg.
TABLE 2.
Total aldehyde and acrolein concentrations of dietary lipid sources1
| Palm oil | Soybean oil | Flaxseed oil | Fish oil | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 90°C for 72 h | Control | 90°C for 72 h | Control | 90°C for 72 h | Control | 90°C for 72 h | |
| EPA, % of total oil | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 16.9 | 14.3 |
| DHA, % of total oil | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 16.6 | 13.2 |
| UFA:SFA | 0.91 | 0.81 | 5.32 | 3.41 | 9.96 | 7.80 | 1.65 | 1.36 |
| Double bonds2 | 57 | 50 | 150 | 126 | 224 | 211 | 212 | 181 |
| AnV3 | 3.9 | 87.0 | 0.8 | 384.1 | ND | ND | 38.4 | 436.6 |
| PV, mEq/kg | 15.1 | 607 | 15.0 | 616 | 11.2 | 128 | 15.0 | 20.4 |
| Aldehydes, mg/kg | 59 | 1327 | 14 | 1553 | 45 | 540 | 177 | 278 |
| Acrolein | 5.8 | 10.4 | 5.5 | 26.6 | 28.9 | 118 | 86.1 | 231 |
| Total tocopherols, mg/kg | 58 | <10 | 802 | 37 | 267 | <10 | 119 | 125 |
Adapted from profiles reported by Lindblom et al. (24). AnV, p-anisidine value; ND, not detectable; PV, peroxide value; UFA:SFA, unsaturated fatty acid:saturated fatty acid ratio.
Double bonds were calculated by multiplying the number of double bonds in a fatty acid by the percentage of that fatty acid detected in the experimental oil, as described by Lindblom et al. (24).
The p-anisidine value is determined by spectrophotometer. In the case of flaxseed oil, color interference from compounds present in flaxseed oil resulted in an undetectable AnV due to background noise. The assay limit of detection was 0.5.
Ileum histomorphology
Ileum samples from 1 bird per cage were fixed in 10% neutral buffered formalin for 24 h at room temperature. Tissues were embedded in paraffin, 3 sections were mounted on a microscope slide, and hematoxylin and eosin stained. Slides were imaged using a DP80 Olympus camera mounted on an OLYMPUS BX 54/43 microscope, and measurements of villus height (VH) and crypt depth (CrD) were taken using the OLYMPUS cell Sens Dimension 1.16 software (Olympus Corporation). VH was measured as the distance from the villus-crypt junction to the villus tip, with the depth of the invagination between adjacent villi being used to measure CrD (49, 50). These measurements were then used to calculate the VH:CrD ratio. For each of the 3 tissue sections mounted on a slide, 15 villi with intact lamina propria were selected and measured resulting in a total of 45 measurements per bird (225 measurements per treatment).
Liver cytokine gene expression
Liver sections were snap frozen in liquid nitrogen, transported on dry ice, and stored at −80°C until analysis. For cytokine gene expression, RNA was isolated and qPCR was performed as previously described (51). Reactions were run in triplicate and a standard curve was generated to estimate the reaction efficiency (slope). Analysis of gene expression was performed as previously described (52). Briefly, the same procedure was used to analyze the expression of 28S ribosomal RNA (RN28S), which was then used to account for the differences in the amount of RNA included in each reaction. Adjusted cycle threshold (Ct) values for statistical analysis were calculated with the following equation:
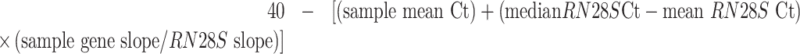 |
(1) |
A higher Ct value indicates greater target gene expression in the sample.
Ileum cytokine expression
The ACDbio RNAscope 2.5 HD Assay-Red kit (Advanced Cell Diagnostics) was used according to the manufacturer's instructions to visualize the RNA content of cytokines and T-cell markers present in the ileum. Briefly, mounted and deparaffinized tissue sections were pretreated with hydrogen peroxide (15 min), boiled in target retrieval buffer (30 min), and pretreated with Protease Plus (Advanced Cell Diagnostics) (30 min). Tissue sections were hybridized with custom species-specific RNAscope probes based on Genbank sequences for 2 h. The bound probe was amplified 6 times for detection of the red signal (probe 2) and amplified an additional 4 rounds to detect the green signal (probe 1) for duplex assays. Probe 1 represented signals for IL1B, IL4, IL6, IL10, and IFNG, whereas probe 2 represented the CD3 signal. CD3 expression was measured on the same tissue section with individual cytokines, whereas CD4 was analyzed as a single stain. Slides were counterstained with hematoxylin and visualized under a standard bright field microscope at 20–40×. The signal dot generated during amplification represented the probe bound to a single RNA molecule. Signal quantification was then performed by HALO analysis software (Indica Labs). The HALO software approximates the location of individual cells based on 4′,6-diamidino-2-phenylindole staining and localizes RNAscope probe signals within a cell. Six fields were quantified per tissue section with sections from 1 bird per cage and 2 birds per treatment (12 measurements per treatment). The HALO software output consisted of the percentage of positive cells for the cytokine or CD3 probe in addition to dual-positive and dual-negative populations. Because signal contributions from dual-positive populations contribute to the overall quantification of an individual probe, data were adjusted to determine the percentage of single-positive cells for each probe. This was done by subtracting the percentage of dual-positive populations contributing to the signal (provided by HALO) from the overall probe signal.
In addition to information about these cell populations, the HALO software outputs a histo-score (H-score) determined by assigning cells to a scored bin based on staining intensity within each probe. Scores range from 0 (no staining) to 4 (high intensity) and the overall score is calculated based on percentages of cells within each bin. Detailed descriptions of HALO software calculations for each cell population and the H-score are included in the Supplemental Methods.
Statistical analysis
Data were analyzed using the following statistical model:
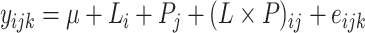 |
(2) |
where yijk is the dependent variable, μ is the overall treatment mean, Li is the main effect of lipid source at the ith level (palm, soybean, flaxseed, or fish), Pj is the main effect of peroxidation status at the jth level (control or peroxidized), L × P is the interaction effect of lipid source and peroxidation status, and eijk is the random error associated with each observation. Two birds per cage were used for cytokine gene expression (n = 10) and 1 bird per cage was used for histomorphology (n = 5). Tissues from 2 birds per treatment were used for RNAscope-ISH, with 6 fields per bird analyzed as repeated measures using a compound symmetry covariance structure and individual bird as the subject. All data were analyzed using the MIXED procedure of SAS 9.4 (SAS Institute), with significance denoted at P ≤ 0.05 and trends noted at 0.05 < P ≤ 0.10. Means ± SEM were reported and separated using LSMEANS with the Tukey adjustment applied to account for multiple comparisons.
Results
Ileum histomorphology
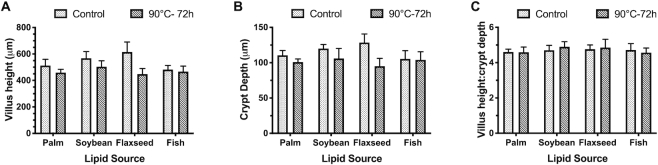
There were no lipid source, peroxidation, or lipid source × peroxidation effects on VH, CrD, or VH:CrD (Figure 1). Peroxidation tended to reduce VH, with birds fed peroxidized oils showing a 13.8% reduced VH compared with birds fed control oils (P = 0.06). CrD also tended to decrease as a result of peroxidation, by 12.6% compared with control oil, regardless of lipid source (P = 0.10).
FIGURE 1.
(A) Villus height, (B) crypt depth, and (C) villus height:crypt depth ratio in the ileum of broiler chickens fed fresh or peroxidized palm, soybean, flaxseed, or fish oils. Data are represented as the mean histomorphological measurement ± individual mean SEM, n = 5 birds.
Liver cytokine gene expression (qPCR)
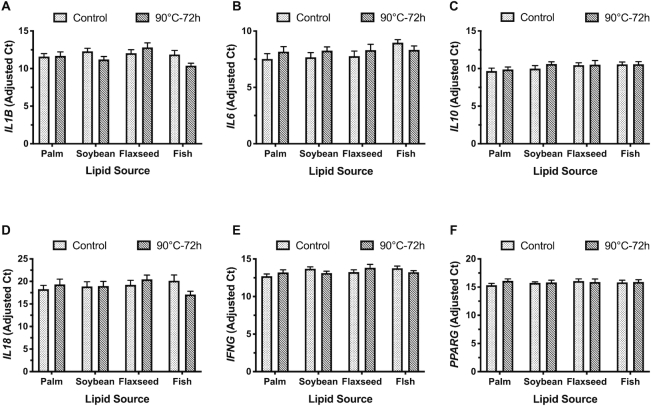
The expression of IL1B tended to increase 1.1-fold in the livers of birds fed flaxseed oil compared with palm and fish oil (P = 0.07). Feeding peroxidized fish oil tended to decrease IL1B expression by 1.1-fold compared with birds fed the corresponding control, thereby driving the trend observed in the lipid × peroxidation effect (P = 0.08). No changes in the liver expression of IL1B, IL6, IL10, IL18, IFNG, or PPARG were observed as a result of lipid source, peroxidation status, or their interaction (Figure 2).
FIGURE 2.
Gene expression of (A) IL1B, (B) IL6, (C) IL10, (D) IL18, (E) IFNG, and (F) PPARG in the liver of broiler chickens fed fresh or peroxidized palm, soybean, flaxseed, or fish oils. Data are presented as the mean adjusted Ct ± individual mean SEM, n = 10 birds, with the exception of control palm oil where n = 8. Ct, cycle threshold; PPARG, peroxisome proliferator–activated receptor-γ.
T-cell markers and cytokine expression (RNAscope-ISH), and CD4+ and ileal CD3+ cell presence
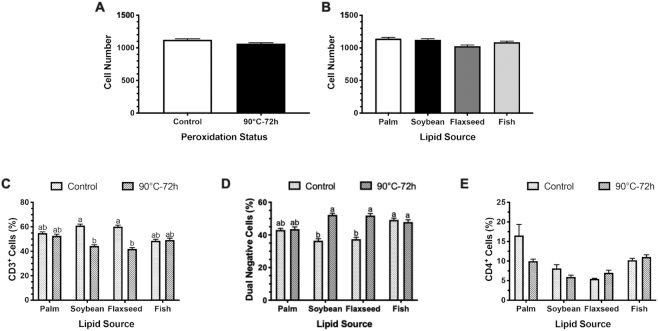
Overall, peroxidation or lipid source did not alter total cell count in visualized fields, but feeding peroxidized oils decreased CD3+ cells by 16.2% without impacting CD4+ populations (P = 0.001; Figure 3). The CD3+ cell loss in the ileum due to peroxidation was coupled with a 15.1% increase in cell populations dual negative for T-cell and cytokine probes in birds fed peroxidized oils (P = 0.006; Figure 3D). In particular, CD3+ expression was reduced by 27.4% and 30.3% only in birds fed peroxidized soybean and flaxseed oils and coupled with 30.3% and 27.8% increased dual-negative cells, respectively, compared with corresponding controls (P = 0.001 and 0.03; Figure 3C, D). While not represented in RNAscope raw data (Figure 4), peroxidation decreased CD3+ cells by 16.8% when data were adjusted for dual positive cell contribution to overall CD3 probe signal (P = 0.0001). Notably, CD3+ cells were consistently reduced by peroxidation, regardless of duplexed cytokine; however, feeding peroxidized soybean and flaxseed oils had the greatest impact on ileal CD3+ cells, resulting in 27.3% and 32.7% reductions, respectively, compared with corresponding controls (P = 0.002; Figures 5–7; Supplemental Figures 1 and 2).
FIGURE 3.
RNAscope analysis of the main effects of (A) peroxidation and (B) lipid source on cell numbers measured by RNAscope in the broiler ileum in addition to populations of (C) CD3+, (D) dual-negative cells, and (E) CD4+ cells. Cell number data represent the mean cell number detected ± SEM, n = 2 birds (36 fields per bird pooled across all cell markers). CD3+ and dual-negative cell data represent the mean percentages ± individual mean SEM, n = 2 birds (mean of 36 scans per bird pooled across duplexed cytokine). CD4 data are the mean ± SEM, n = 2 birds (mean of 6 scanned fields per bird). Labeled means without a common letter differ, P ≤ 0.05.
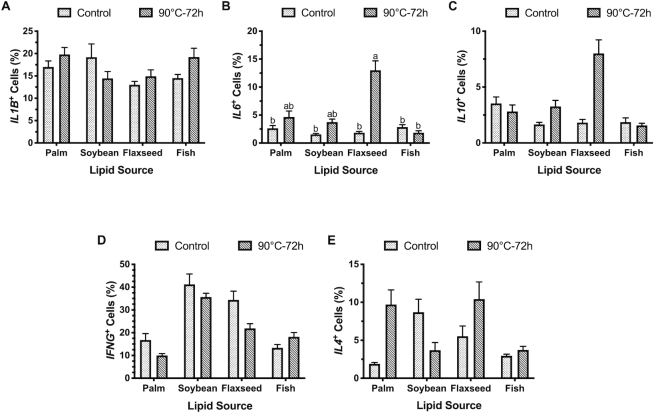
FIGURE 4.
Percentage of cells positive for (A) IL1B, (B) IL6, (C) IL10, (D) IFNG, and (E) IL4 in the ileum of broiler chickens fed fresh or peroxidized palm, soybean, flaxseed, or fish oils. Data represent the mean percentage of cells positive for each cytokine or T-cell marker ± individual mean SEM, n = 2 birds per treatment (6 scanned fields per bird). Labeled means without a common letter differ, P ≤ 0.05.
FIGURE 5.
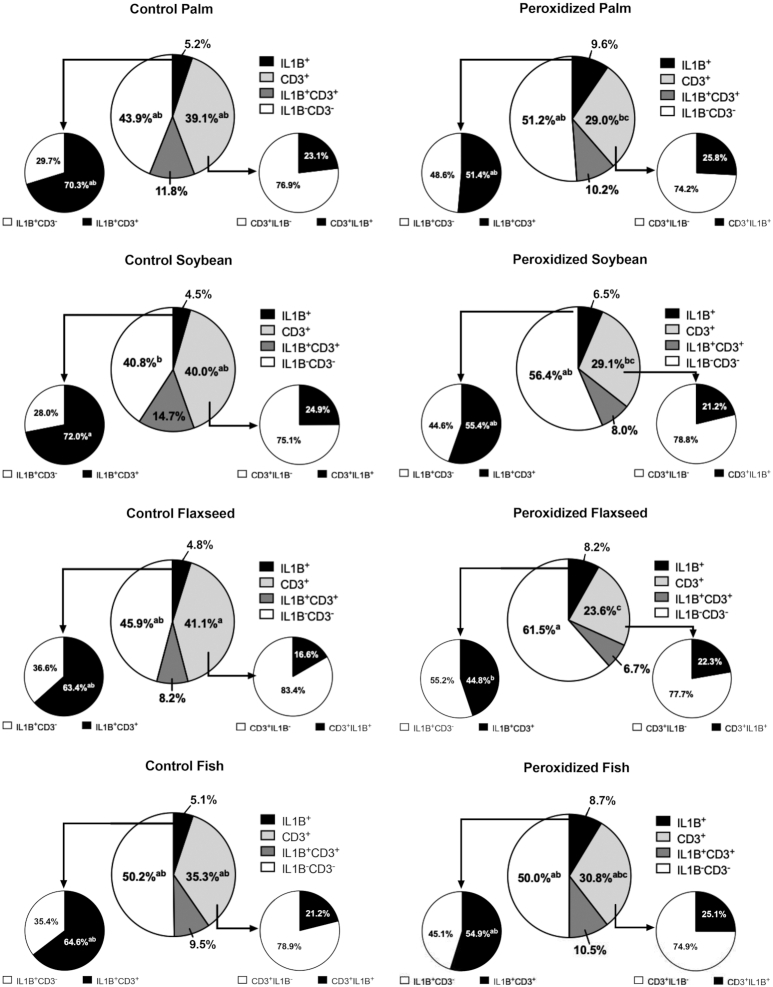
Percentages of IL1B+, CD3+, IL1B+CD3+, and IL1B−CD3− cells in the ileum of broiler chickens fed fresh or peroxidized palm, soybean, flaxseed, or fish oils. The percentage of cells positive for the IL1B probe that were also CD3+ are represented by the pie chart connected to the IL1B+ slice. Likewise, the percentage of cells positive for the CD3 probe that were also IL1B+ are represented by the pie chart connected to the CD3+ slice. Data represent the mean percentage of cells positive for IL1B or CD3, n = 2 birds (6 scanned fields per bird). All cell populations within each treatment are presented for clarity. Due to the complex nature of this presentation, statistical comparison of each cell population is made between treatments rather than within treatment. As such, different superscripts within the same cell population (i.e., the same-colored slice between treatments) indicate significant differences, P ≤ 0.05.
FIGURE 7.
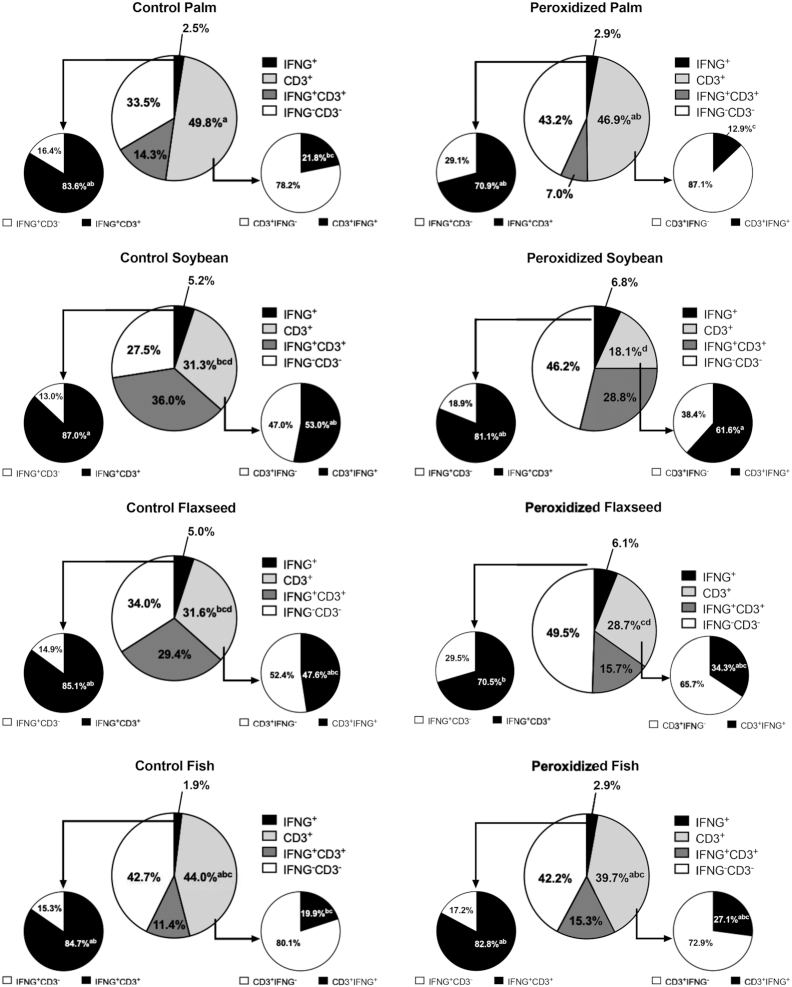
Percentages of IFNG+, CD3+, IFNG+CD3+, and IFNG−CD3− cells in the ileum of broiler chickens fed fresh or peroxidized palm, soybean, flaxseed, or fish oils. The percentage of cells positive for the IFNG probe that were also CD3+ are represented by the pie chart connected to the IFNG+ slice. Likewise, the percentage of cells positive for the CD3 probe that were also IFNG+ are represented by the pie chart connected to the CD3+ slice. Data represent the mean percentage of cells positive for IFNG or CD3, n = 2 birds (6 scanned fields per bird). All cell populations within each treatment are presented for clarity. Due to the complex nature of this presentation, statistical comparison of each cell population is made between treatments rather than within treatment. As such, different superscripts within the same cell population (i.e., the same-colored slice between treatments) indicate significant differences, P ≤ 0.05.
Ileum cytokine production
In raw RNAscope data, feeding peroxidized oils increased IL6+ cells by 62.0% (P = 0.02), but no changes to IL1B, IL10, or IL4+ cells were observed (Figure 4). Production of IL6 was most impacted by flaxseed oil inclusion, with 51.0–68.5% greater ileal expression compared with other oils (P = 0.05; Figure 4). This effect was heightened by feeding peroxidized flaxseed oil, resulting in the greatest percentage of ileal IL6+ cells (13.0% compared with 1.5–4.7%) with an 85.8% difference between peroxidized and control flaxseed oil (P = 0.03; Figure 4B). In contrast, lipid source had greater impacts on ileal IFNG production, where soy oil supplementation increased IFNG+ cells by 59.1% and 65.3% compared with birds fed fish and palm oils, respectively, regardless of peroxidation (P = 0.01; Figure 4D).
When data were adjusted for the percentage of IL1B+CD3+ cells contributing to individual IL1B+ and CD3+signals, peroxidation increased IL1B-producing cells by 40.3%, with additional 17.4% increases in IL1B−CD3− cells (P = 0.01 and 0.006). Compared with birds fed control oils, those fed peroxidized oils had a 23.6% reduction in IL1B+CD3+ cells contributing to the overall IL1B+ signal (P = 0.001). Generally, these results indicate that IL1B production in the broiler ileum was more sensitive to peroxidation than lipid source. The shift in dual-positive cells between control and peroxidized oils suggests that CD3+ cells are partially responsible for IL1B production, with another cell type likely contributing (Figure 5).
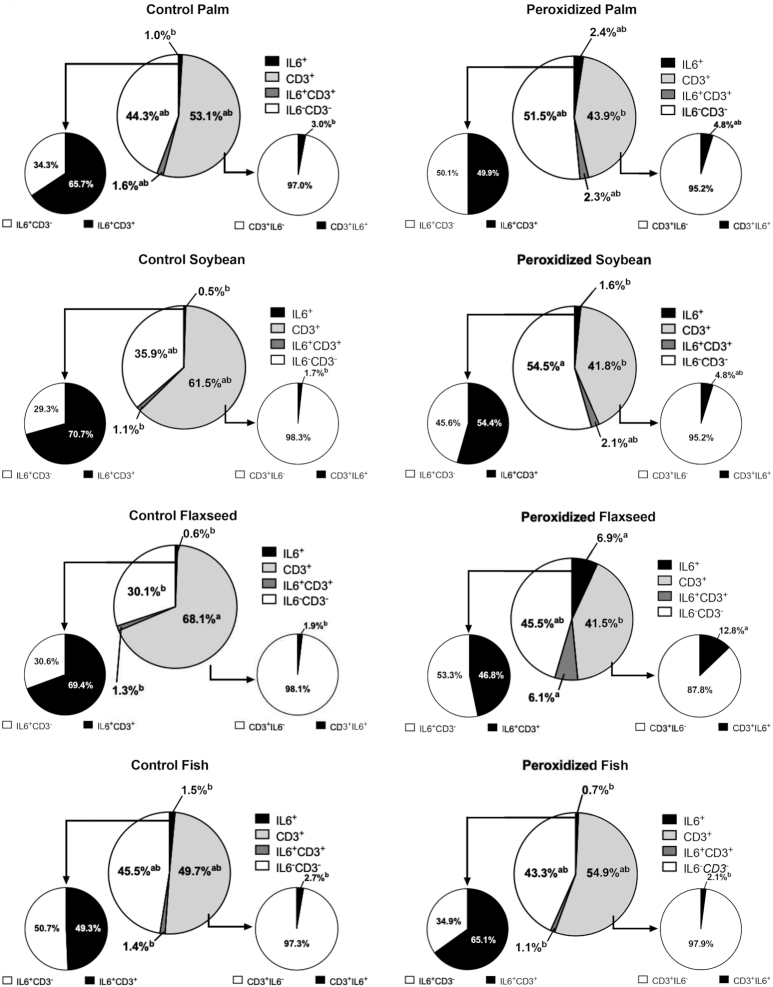
In data adjusted to remove dual-positive cell contributions to IL6+ and CD3+ signals, peroxidation increased ileal IL6 by 70.0% (P = 0.01). Specifically, ileal IL6 production was most affected by peroxidized flaxseed oil, with birds fed this diet having 91.8% more IL6+ cells compared with the corresponding control (P = 0.02). Whereas feeding peroxidized oils increased overall IL6+CD3+ cells by 53.8% (P = 0.02), feeding peroxidized flaxseed oil tended to increase these cells by 79.0% compared with the corresponding control (P = 0.06). Regardless of source, peroxidized oils increased cell types expressing neither IL6 nor CD3 by 18.4% (P = 0.02). Feeding peroxidized oils increased CD3+IL6+cells by 62.3% (P = 0.01), but tended to decrease CD3+ cells contributing to the IL6 probe signal by 15.2% (P = 0.10). In keeping with general observations, feeding peroxidized flaxseed oil increased populations of CD3+IL6+ cells by 85.3% compared with the corresponding control (P = 0.05). The shift in cell surface expression between control and peroxidized oils suggests that the remaining CD3+ cells were predominantly responsible for increased ileal IL6 (1.9–12.8%; P = 0.05; Figure 6).
FIGURE 6.
Percentages of IL6+, CD3+, IL6+CD3+, and IL6−CD3− cells in the ileum of broiler chickens fed fresh or peroxidized palm, soybean, flaxseed, or fish oils. The percentage of cells positive for the IL6 probe that were also CD3+ are represented by the pie chart connected to the IL6+ slice. Likewise, the percentage of cells positive for the CD3 probe that were also IL6+ are represented by the pie chart connected to the CD3+ slice. Data represent the mean percentage of cells positive for IL6 or CD3, n = 2 birds (6 scanned fields per bird). All cell populations within each treatment are presented for clarity. Due to the complex nature of this presentation, statistical comparison of each cell population is made between treatments rather than within treatment. As such, different superscripts within the same cell population (i.e., the same-colored slice between treatments) indicate significant differences, P ≤ 0.05.
After adjusting for the presence of dual-positive cells contributing to IFNG and CD3 signals, ileal tissue from birds fed soybean oil–enriched diets expressed 48.8% and 40.9% fewer CD3+ cells compared with palm and fish oils, respectively (P = 0.0002; Figure 7). In contrast to changes in IL6 production from feeding peroxidized flaxseed oil, feeding soybean oil increased IFNG by 55.1% and 59.9% compared with palm and fish oil, respectively (P = 0.007; Figure 7). Soybean oil also increased IFNG+CD3+ cells 67.1% and 58.9% compared with birds fed palm and fish oils, respectively (P = 0.02; Figure 7). Feeding peroxidized oils increased dual-negative cells by 24.0% (P = 0.01) and contributed to concurrent 10.3% reductions in overall IFNG+CD3+ cells compared with birds fed control oils (P = 0.003). Lipid source tended to increase dual-positive cells within IFNG+ cell populations, with soybean and fish oils increasing IFNG+CD3+ cells by 6.0–6.8% compared with palm and flaxseed oil (P = 0.08; Figure 7). Feeding soybean and flaxseed oils resulted in 69.8% and 57.7% greater IFNG+CD3+ cells within the CD3+ population, respectively, than birds fed palm oil (P = 0.002; Figure 7). Shifts in dual-negative populations as well as increased IFNG+ cells suggests a wider range of cell types contributing to IFNG production that are more responsive to lipid source than peroxidation.
Whereas IL1B, IL6, and IFNG were differentially impacted by lipid source or peroxidation, only trends were noted for IL10. In adjusted data, IL10-producing cells tended to increase by 59.4% in birds fed peroxidized lipids, whereas IL10−CD3− cells tended to decrease by 12.5% (P = 0.07). Peroxidation also tended to decrease dual-positive cells contributing to IL10+ cell populations by 20.0% (P = 0.08). Notably, feeding peroxidized flaxseed oil tended to increase IL10+ populations by 88.1% compared with the corresponding control (P = 0.1; Supplemental Figure 1). No changes in IL4+ cell populations were observed as a result of lipid source or peroxidation status (Supplemental Figure 2).
When examining the H-score data, the maximum recorded H-score was 104.8, indicating an overall low staining intensity, with the majority of cells being assigned to bins 0–2 (Supplemental Figures 3–10). Peroxidation decreased the overall H-score of CD3+ cells (P = 0.002), with more specific 35.0- and 41.5-point reductions observed in the CD3+ H-score of birds fed peroxidized soybean and flaxseed oils compared with corresponding controls (P = 0.007; Supplemental Figure 4). In cytokine-producing cells, responses to lipid source and peroxidation varied between cytokines. All 3 factors impacted overall H-score and bin composition of IL6+ cells, whereas lipid source had greater impacts on IFNG+ cells. More specific analysis of cell compositions within scored bins and the impacts of lipid source and peroxidation on measured H-scores can be found in the Supplemental Results.
Discussion
A concern with feeding heated oils to livestock is the histomorphological and inflammatory state changes in digestive tissues, with varying results observed across publications and animal models (25, 27, 53–56). The ileum was selected for analysis due to its proximity to immune structures (cecal tonsils) and the comparatively slower transit time, which increases tissue exposure to any peroxidized lipid remaining in the digesta (57). Although histomorphological measurements taken here were consistent with those reported by Awad et al. (49), we found a limited ileal histomorphological response to lipid source or peroxidation. Feeding broilers peroxidized poultry fat previously resulted in increased intestinal epithelial cell turnover (histomorphology not evaluated) (53). Other reports also observed no significant changes to ileal morphology in response to feeding different lipid sources to broilers, which aligns with the results reported herein (58, 59).
Whereas the main effect of peroxidation generally reduced CD3+ T-cells in all duplexed assays, peroxidized soybean and flaxseed oils contributed to greater reductions in these populations. In contrast, fish and palm oil diets generally maintained T-cell populations regardless of peroxidation (Figure 3C). Local cytokine responses to lipid source and peroxidation in the broiler ileum were primarily observed in diets containing peroxidized soybean and flaxseed oils (Figure 4A–D). In the corresponding performance publication for this study, it was noted that birds fed peroxidized soybean and flaxseed oil had reduced average daily gain and feed intake (24). Taken together, these findings suggest that oils with higher susceptibility to peroxidation contribute to greater immunological changes, which could result in reduced broiler performance.
When considering global immune cell population changes, tissues isolated from birds fed fish oil contained remarkably constant populations compared with all other treatments, even when peroxidized (Figures 5 –7). In contrast, significant changes in CD3+ T-cell presence and cytokine production were observed when comparing peroxidized and control flaxseed oil. When considering changes to the oil compositions before and after heating, fish oil composition did not change as drastically as other oils and maintained EPA/DHA concentrations (for a more detailed breakdown, see reference 24). Flaxseed oil accumulated the highest and most bioreactive peroxidation products, which could have influenced immunological changes and contributed to increased IL6 production (Table 2). We postulate that stability of immune cell populations and associated cytokine production in fish oil diets, in contrast to an increased immunological response to peroxidized flaxseed oil, likely has to do with the preservation of biologically active EPA/DHA (Table 2) (9, 46, 60).
Previous reports also show that thermally processed flaxseed and fish oils contained high concentrations of acrolein, accounting for 21.9% and 83.1% of the aldehydes measured in peroxidized flaxseed and fish oil, respectively (24) (Table 2). The effects of feeding acrolein to poultry are not well documented; however, its immunomodulatory effects have been studied in various models. Decreased production of IL-2 and other cytokines was observed in human T-cells cultured with 2.5 μmol/L acrolein, indicating that the compound has an inhibitory effect on T-cell responses (61). This corresponds to the decreased T-cell presence observed in this study, but the mechanisms responsible for this observation were not offered by previous research. When administered orally, acrolein increased ileal CrD and intestinal permeability in mice, which is inconsistent with the lack of changes shown here (62). Notably, changes to IL6 and other proinflammatory cytokines were seen in birds fed peroxidized flaxseed oil but not in birds fed peroxidized fish oil, despite a higher acrolein content. In addition to the protective effect of EPA/DHA, our concurrent performance work reported that birds fed control and peroxidized fish oil ate significantly less than other treatments, which might additionally explain the differences in acrolein response between these 2 groups (24).
Lipid source had significant impacts on IFNG-producing cells in the broiler ileum, whereas peroxidation did not impact these populations. Previous work also reported that feeding peroxidized soybean oil had no effect on the relative abundance of IFNG transcripts in the broiler jejunum (16). Within the context of this study, the similarly high production of IFNG in birds fed soybean and flaxseed oil contradicts classification of n–6 PUFAs and n–3 PUFAs as pro- and anti-inflammatory, respectively. Studies utilizing qPCR to analyze cytokine alterations due to lipid source in broilers similarly observed increased IFNG expression in the serum and spleen in response to supplementation with n–3 PUFAs (14, 63). The soybean oil used in this study had higher amounts of linoleic acid compared with the other oils (24); however, compounds present in soybean oil other than linoleic acid might be responsible for the significantly greater production of IFNG observed in the ileum of broilers fed soybean oil.
The observed inflammation due to lipid source and peroxidation could also be localized to ileal tissue, because liver cytokine expression was unchanged and might represent a more systemic view of immune activation (64). Definitive explanations for changes in ileal T-cell populations as a result of feeding peroxidized lipids cannot be described by the results of this study because only CD3 and CD4 markers were analyzed. The decreased presence of T-cells in broilers fed peroxidized oils suggests that these cells are either leaving the tissue or recruitment to the ileum is impaired. In rats, feeding heated soybean and palm oils increased expression of vascular cell adhesion molecule (VCAM)-1 in the aorta, which facilitates immune cell migration into tissue (30, 31, 65). In endothelial cell culture, IL-1 and IL-6 had no effect on mouse lymphocyte migration, whereas IFN-γ inhibited T-cell migration at high doses despite no changes to VCAM-1 expression (66). Decreased T-cell presence in the ileum of broilers fed peroxidized soybean and flaxseed oil might be due to the observed increase in IFNG, but increased IL6 due to peroxidized flaxseed oil likely does not have an additive effect on impaired T-cell migration. These alterations in lymphocyte migration have not been well documented in vivo and are not well defined in avian models, emphasizing a need for further research into the effect of lipid source and peroxidation on immune cell migration in broilers.
Within the context of this study, lipid source and peroxidation did not have an effect on intestinal histomorphology or liver cytokine profiles analyzed by qPCR as a marker for systemic immunity. The reported findings ultimately suggest that broiler immune responses to varying lipid sources and peroxidation are primarily localized to intestinal tissues after 21 d without grossly impacting intestinal structure. However, there might be unintended consequences associated with feeding yellow grease to broiler chickens due to the level of peroxidation and lipid composition. Generally, yellow grease with lipid peroxidation profiles equivalent to heating at 90°C for 72 h can impair T-cell recruitment and reduce the ability of birds to adequately respond to the environmental and immune challenges associated with broiler production (e.g., bacteria, parasites, or heat stress). Increased IL6 production in response to peroxidized flaxseed oil and accompanying reductions in performance reported by Lindblom et al. (24) further indicate that yellow grease with a high percentage of flaxseed oil should be avoided in broiler diets due to increased inflammation in healthy animals. Performance outcomes suggest that feeding fresh soybean oil might not impair broiler health because the increased IFNG does not affect bird performance. It is important to note that combined IFNG production and impaired T-cell populations from peroxidized soybean oil present in yellow grease might negatively impact production and requires further evaluation (24).
RNAscope as a method of analyzing immune parameters in the ileum provided a greater depth of knowledge by utilizing basic T-cell markers to identify cell populations potentially responsible for changes in cytokine production associated with dietary lipids. The potential for application of several cell markers to RNAscope-ISH makes it a useful tool for further studies on the immune responses to dietary lipid source and peroxidation in broiler chickens.
Supplementary Material
Acknowledgments
We thank the staff at the Iowa State University Poultry Research and Teaching Farm for animal care during the trial, and the Iowa State Veterinary Pathology laboratory for assistance with RNAscope assays.
The authors’ responsibilities were as follows—SCL, BJK, and EAB: designed and conducted the research and collected data; KAF-C and MMM: participated in data analysis and interpretation; KAF-C, BJK, and EAB: wrote the paper; EAB: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Support for this work was provided by internal funding.
Author disclosures: The authors report no conflicts of interest.
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by Iowa State University or the USDA and does not imply approval to the exclusion of other products that may be suitable. The USDA is an equal opportunity provider and employer.
Supplemental Methods, Supplemental Results, Supplemental Table 1, and Supplemental Figures 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CrD, crypt depth; Ct, cycle threshold; FA, fatty acid; H-score, histo-score; ISH, in situ hybridization; PPARG, peroxisome proliferator–activated receptor γ; UFA, unsaturated fatty acid; VCAM, vascular cell adhesion molecule; VH, villus height.
Contributor Information
Krysten A Fries-Craft, Department of Animal Science, Iowa State University, Ames, IA, USA.
Meaghan M Meyer, Department of Animal Science, Iowa State University, Ames, IA, USA.
Stephanie C Lindblom, Department of Animal Science, Iowa State University, Ames, IA, USA.
Brian J Kerr, USDA-ARS-National Laboratory for Agriculture and the Environment, Ames, IA, USA.
Elizabeth A Bobeck, Department of Animal Science, Iowa State University, Ames, IA, USA.
References
- 1. Enos R, Davis J, Velazquez K, McClellan J, Carnevale K, Murphy E. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54:152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kennedy A, Martinez K, Chuang C-C, Lapoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4. [DOI] [PubMed] [Google Scholar]
- 3. Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–7. [DOI] [PubMed] [Google Scholar]
- 4. Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. [DOI] [PubMed] [Google Scholar]
- 5. Fritsche KL. The science of fatty acids and inflammation. Adv Nutr. 2015;6:293S–301S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calder PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. 2015;39:18S. [DOI] [PubMed] [Google Scholar]
- 7. Lee SA, Whenham N, Bedford MR. Review on docosahexaenoic acid in poultry and swine nutrition: consequence of enriched animal products on performance and health characteristics. Anim Nutr. 2019;5:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svahn SL, Ulleryd MA, Grahnemo L, Ståhlman M, Borén J, Nilsson S, Jansson J-O, Johansson ME. Dietary omega-3 fatty acids increase survival and decrease bacterial load in mice subjected to Staphylococcus aureus-induced sepsis. Infect Immun. 2016;84:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105–15. [DOI] [PubMed] [Google Scholar]
- 10. Harizi H, Corcuff J-B, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–9. [DOI] [PubMed] [Google Scholar]
- 11. Duda MK, O'Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG et al. . Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovas Res. 2009;81:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calder PC. n–3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–19S. [DOI] [PubMed] [Google Scholar]
- 13. Vaughan RA, Garrison RL, Stamatikos AD, Kang M, Cooper JA, Paton CM. A high linoleic acid diet does not induce inflammation in mouse liver or adipose tissue. Lipids. 2015;50:1115–22. [DOI] [PubMed] [Google Scholar]
- 14. Ibrahim D, El-Sayed R, Khater SI, Said EN, El-Mandrawy SAM. Changing dietary n-6:n-3 ratio using different oil sources affects performance, behavior, cytokines mRNA expression and meat fatty acid profile of broiler chickens. Anim Nutr. 2018;4:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu P, Kerr BJ, Weber TE, Chen C, Johnston LJ, Shurson GC. Influence of thermally oxidized vegetable oils and animal fats on intestinal barrier function and immune variables in young pigs. J Anim Sci. 2014;92:2971–9. [DOI] [PubMed] [Google Scholar]
- 16. Liang F, Jiang S, Mo Y, Zhou G, Yang L. Consumption of oxidized soybean oil increased intestinal oxidative stress and affected intestinal immune variables in yellow-feathered broilers. Asian Australas J Anim Sci. 2015;28:1194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Awada M, Soulage CO, Meynier A, Debard C, Plaisancié P, Benoit B, Picard G, Loizon E, Chauvin M-A, Estienne M et al. . Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J Lipid Res. 2012;53:2069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanner J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol Nutr Food Res. 2007;51:1094–101. [DOI] [PubMed] [Google Scholar]
- 19. Ouart MD, Damron BL, Martin FG, Christmas RB, Sloan DR. Effects of poultry fat and yellow grease on broiler performance and profitability. Poult Sci. 1992;71:821–8. [Google Scholar]
- 20. Pesti GM, Bakalli RI, Qiao M, Sterling KG. A comparison of eight grades of fat as broiler feed ingredients. Poult Sci. 2002;81:382–90. [DOI] [PubMed] [Google Scholar]
- 21. Lin CF, Asghar A, Gray JI, Buckley DJ, Booren AM, Crackel RL, Flegal CJ. Effects of oxidised dietary oil and antioxidant supplementation on broiler growth and meat stability. Br Poult Sci. 1989;30:855–64. [DOI] [PubMed] [Google Scholar]
- 22. Wu H, Gong LM, Guo L, Zhang LY, Li JT. Effects of the free fatty acid content in yellow grease on performance, carcass characteristics, and serum lipids in broilers. Poult Sci. 2011;90:1992–8. [DOI] [PubMed] [Google Scholar]
- 23. Ehr IJ, Kerr BJ, Persia ME. Effects of peroxidized corn oil on performance, AMEn, and abdominal fat pad weight in broiler chicks. Poult Sci. 2015;94:1629–34. [DOI] [PubMed] [Google Scholar]
- 24. Lindblom SC, Gabler NK, Bobeck EA, Kerr BJ. Oil source and peroxidation status interactively affect growth performance and oxidative status in broilers from 4 to 25 d of age. Poult Sci. 2019;98:1749–61. [DOI] [PubMed] [Google Scholar]
- 25. Lindblom SC, Gabler NK, Kerr BJ. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in growing pigs. J Anim Sci. 2018;96:558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindblom SC, Gabler NK, Dilger RN, Olson ZF, Loving CL, Kerr BJ. Influence of feeding thermally peroxidized soybean oil on oxidative status in growing pigs. J Anim Sci. 2018;96:545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Overholt MF, Dilger AC, Boler DD, Kerr BJ. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in finishing pigs. J Anim Sci. 2018;96:2789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Porcelli SA, Segelke BW, Sugita M, Wilson IA, Brenner MB. The CD1 family of lipid antigen-presenting molecules. Immunol Today. 1998;19:362–8. [DOI] [PubMed] [Google Scholar]
- 29. Hörkkö S, Binder CJ, Shaw PX, Chang M-K, Silverman G, Palinski W, Witztum JL. Immunological responses to oxidized LDL. Free Radic Biol Med. 2000;28:1771–9. [DOI] [PubMed] [Google Scholar]
- 30. Ng C-Y, Kamisah Y, Faizah O, Jubri Z, Qodriyah HMS, Jaarin K. Involvement of inflammation and adverse vascular remodelling in the blood pressure raising effect of repeatedly heated palm oil in rats. Int J Vasc Med. [Internet]2012;2012:404025 Available from: 10.1155/2012/404025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ng C-Y, Kamisah Y, Faizah O, Jaarin K. The role of repeatedly heated soybean oil in the development of hypertension in rats: association with vascular inflammation. Int J Exp Path. 2012;93:377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang YW, Field CJ, Sim JS. Dietary polyunsaturated fatty acids alter lymphocyte subset proportion and proliferation, serum immunoglobulin G concentration, and immune tissue development in chicks. Poult Sci. 2000;79:1741–8. [DOI] [PubMed] [Google Scholar]
- 33. Korver DR, Klasing KC. Dietary fish oil alters specific and inflammatory immune responses in chicks. J Nutr. 1997;127:2039–46. [DOI] [PubMed] [Google Scholar]
- 34. Korver DR, Roura E, Klasing KC. Effect of dietary energy level and oil source on broiler performance and response to an inflammatory challenge. Poult Sci. 1998;77:1217–27. [DOI] [PubMed] [Google Scholar]
- 35. Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–8. [DOI] [PubMed] [Google Scholar]
- 36. Al-Khalifa H, Givens DI, Rymer C, Yaqoob P. Effect of n-3 fatty acids on immune function in broiler chickens. Poult Sci. 2012;91:74–88. [DOI] [PubMed] [Google Scholar]
- 37. Su S, Dwyer DM, Miska KB, Fetterer RH, Jenkins MC, Wong EA. Expression of host defense peptides in the intestine of Eimeria-challenged chickens. Poult Sci. 2017;96:2421–7. [DOI] [PubMed] [Google Scholar]
- 38. Hihi AK, Michalik L, Wahli W. PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci. 2002;59:790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. [DOI] [PubMed] [Google Scholar]
- 40. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 42. Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. [DOI] [PubMed] [Google Scholar]
- 43. Akira S. The role of IL-18 in innate immunity. Curr Opin Immunol. 2000;12:59–63. [DOI] [PubMed] [Google Scholar]
- 44. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. [DOI] [PubMed] [Google Scholar]
- 45. Cunnane SC, Ganguli S, Menard C, Liede AC, Hamadeh MJ, Chen ZY, Wolever TM, Jenkins DJ. High alpha-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr. 1993;69:443–53. [DOI] [PubMed] [Google Scholar]
- 46. Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–22. [DOI] [PubMed] [Google Scholar]
- 48. National Research Council Nutrient requirements of poultry. 9th rev. ed Washington (DC): The National Academies Press; 1994. [Google Scholar]
- 49. Awad WA, Ghareeb K, Abdel-Raheem S, Bohm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. 2009;88:49–56. [DOI] [PubMed] [Google Scholar]
- 50. Rebole A, Ortiz LT, Rodriguez ML, Alzueta C, Trevino J, Velasco S. Effects of inulin and enzyme complex, individually or in combination, on growth performance, intestinal microflora, cecal fermentation characteristics, and jejunal histomorphology in broiler chickens fed a wheat- and barley-based diet. Poult Sci. 2010;89:276–86. [DOI] [PubMed] [Google Scholar]
- 51. Cheeseman JH, Kaiser MG, Ciraci C, Kaiser P, Lamont SJ. Breed effect on early cytokine mRNA expression in spleen and cecum of chickens with and without Salmonella enteritidis infection. Dev Comp Immunol. 2007;31:52–60. [DOI] [PubMed] [Google Scholar]
- 52. Coble DJ, Redmond SB, Hale B, Lamont SJ. Distinct lines of chickens express different splenic cytokine profiles in response to Salmonella enteritidis challenge. Poult Sci. 2011;90:1659–63. [DOI] [PubMed] [Google Scholar]
- 53. Dibner JJ, Atwell CA, Kitchell ML, Shermer WD, Ivey FJ. Feeding of oxidized fats to broilers and swine: effects on enterocyte turnover, hepatocyte proliferation and the gut associated lymphoid tissue. Anim Feed Sci Technol. 1996;62:1–13. [Google Scholar]
- 54. Rosero DS, Odle J, Moeser AJ, Boyd RD, van Heugten E. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br J Nutr. 2015;114:1985–92. [DOI] [PubMed] [Google Scholar]
- 55. Varady J, Eder K, Ringseis R. Dietary oxidized fat activates the oxidative stress-responsive transcription factors NF-κB and Nrf2 in intestinal mucosa of mice. Eur J Nutr. 2011;50:601–9. [DOI] [PubMed] [Google Scholar]
- 56. Ringseis R, Piwek N, Eder K. Oxidized fat induces oxidative stress but has no effect on NF-κB-mediated proinflammatory gene transcription in porcine intestinal epithelial cells. Inflamm Res. 2007;56:118–25. [DOI] [PubMed] [Google Scholar]
- 57. Lillehoj HS, Trout JM. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev. 1996;9:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdulla NR, Loh TC, Akit H, Sazila AQ, Foo HL. Effects of dietary oil sources and calcium:phosphorus levels on growth performance, gut morphology and apparent digestibility of broiler chickens. S Afr J Anim Sci. 2016;46:42–53. [Google Scholar]
- 59. Khatun J, Loh TC, Akit H, Foo HL, Mohamad R. Influence of different sources of oil on performance, meat quality, gut morphology, ileal digestibility and serum lipid profile in broilers. J Appl Anim Res. 2018;46:479–85. [Google Scholar]
- 60. Verlengia R, Gorjão R, Kanunfre C, Bordin S, Lima T, Martins E, Newsholme P, Curi R. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids. 2004;39:857–64. [DOI] [PubMed] [Google Scholar]
- 61. Lambert C, McCue J, Portas M, Ouyang Y, Li J, Rosano TG, Lazis A, Freed BM. Acrolein in cigarette smoke inhibits T-cell responses. J Allergy Clin Immunol. 2005;116:916–22. [DOI] [PubMed] [Google Scholar]
- 62. Chen W-Y, Wang M, Zhang J, Barve SS, McClain CJ, Joshi-Barve S. Acrolein disrupts tight junction proteins and causes endoplasmic reticulum stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am J Pathol. 2017;187:2686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maroufyan E, Kasim A, Ebrahimi M, Loh TC, Bejo MH, Zerihun H, Hosseni F, Goh YM, Farjam AS. Omega-3 polyunsaturated fatty acids enrichment alters performance and immune response in infectious bursal disease challenged broilers. Lipids Health Dis. 2012;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol Immunol. 2002;39:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tudor K-SRS, Hess KL, Cook-Mills JM. Cytokines modulate endothelial cell intracellular signal transduction required for VCAM-1-dependent lymphocyte transendothelial migration. Cytokine. 2001;15:196–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.