Abstract
Introduction
The combination of stromal vascular fraction (SVF) and platelet-rich plasma (PRP) is effective at increasing the concentration of growth factors including transforming growth factor-β (TGF-β). The increase in this growth factor is expected to accelerate the healing of burns. This study aims to determine the effect of giving a combination of SVFs and PRP on TGF-β levels in the healing process of deep dermal burns.
Methods
This was an experimental study in 64 rats using a post-test control group design consisting of 1 group of SVFs and PRP combination injection treatment group, 1 group given a topical combination of SVFs and PRP, 1 group given Vaseline, and 1 control group.
Results
There was a significant difference in TGF-β levels between the deep dermal burns group that was given a combination of SVFs and PRP injection and topical, the Vaseline group, and the control group with p-value <0.05.
Conclusion
The combination of SVFs and PRP increases the level of TGF-β in the healing process of deep dermal burns.
Keywords: TGF-β, Stromal vascular fraction, Platelet-rich plasma, Burn injury, Experimental research
Highlights
-
•
The combination of SVFs and PRP is effective at increasing the concentration of growth factors.
-
•
Administration of SVFs and PRP by injection was not better than topical at increasing the expression of TGF-β.
-
•
The combination of SVFs and PRP increased the level of TGF-β in the healing process rats model.
1. Introduction
Burns are defined as damage to the skin and underlying tissue caused by heat, chemicals, or electricity [[1], [2], [3], [4]]. Stromal vascular fraction (SVF) is a component of lipoaspirates obtained from fat tissue liposuction. Lipoaspirates contain a large number of stem cells called adipose-derived stem cells (ASCs) [[5], [6], [7]]. SVFs are processed in such a way that they contain a consistent, reproducible heterogeneous cell composition. After the production and recording process, SVFs derived from adipose can differentiate into different tissue types, support neovascularization, replace cells, and repair injured tissue [6,8,9].
Platelet-rich plasma (PRP) is a concentrated platelet in small volume plasma, which contains at least six major growth factors, including platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), and transforming growth factor-b (TGF-b), which are released after platelet activation [5,7,[9], [10], [11], [12], [13], [14]].
TGF-β plays a physiological role by binding to TGF-β receptors (TGF-βR) and regulating developmental pathways, controlling the pattern of mesoderm changes in the early embryonic period through the development of several organ systems [[15], [16], [17]]. TGF-β in the initial wound-healing process is released by activated monocytes to stimulate monocytes to express several other peptides such as TGF-α, IL-1, and PDGF. Apart from being released by monocytes, TGF-β is also released by platelets and fibroblasts in the wound [[15], [16], [17], [18]].
This study aims to determine the effect of stem cell combination (SVFs and PRP) on TGF-β levels in the healing process of deep dermal burns in a rats model.
2. Methods
Sixty-four male Sprague-Dawley rats aged 10 weeks, range of weight 150–250 g, were used in this animal experimental study. These experimental animals were fed BR-1 without additional feeding for approximately 1 week for the adaptation period. Feeding took place daily, with aquadest provided as a drink. The cage was standard shaped, cleaned regularly, and given lighting (12 h light/dark photoperiod). Temperature was kept at 24 ± 2 °C. This study was conducted in the animal laboratory Faculty of Medicine, Hasanuddin University, and was approved by the Ethics Commission registration number: 1029/UN4.6.4.5.31/PP36/2019. The work was also carried out in line with the ARRIVE guidelines for reporting animal research [19,20].
Rats were randomly assigned to four groups: 3 treatment groups (sacrificed 1, 4, 7, 10, and 14 days post treatment) and 1 control group (sacrificed day 0). Group A was a control group of TGF levels in healthy rats without treatment. Group B was a control group of rats that were modelled deep dermal burns and Vaseline wound care. Group C was a treatment group given topical PRP + SVFs. Group D was a treatment group of mice given PRP + SVFs injection. PRP from venous blood and SVF from lipoaspirate from the donor mouse were harvested and processed according to Juntendo University School of Medicine, Japan established protocols [21].
2.1. PRP preparation
The rats were shaved on their backs, then ether was used as an inhalation anesthetic. The donor group underwent a thoracotomy until the heart was visible. Apex identification was then performed by aspirating blood from the apex using a 3 cc 25G needle. The blood drawn was transferred to a tube containing 3.8% sodium citrate. Blood was centrifuged for 10 min at 2400 rpm (450 g) for the first centrifugation. The supernatant plasma with a buffy coat was collected and centrifuged at 3600 rpm (850 g) for 15 min. The top layer of plasma was discarded until 5 mL of plasma remained at the bottom. The remaining plasma was collected as PRP, frozen at −20 °C, and thawed before use [22].
2.2. SVF preparation
SVF preparation was derived from adipose tissue from the inguinal portion of rat donors.The fat taken was washed with phosphate-buffered saline (PBS) (Gibco-BRL, Grand Island, NY, USA), chopped into small pieces until smooth, then digested with 0.15% collagenase (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and centrifuged for 30 min at 37 in a 50 mL centrifuge tube. Dulbecco's Modified Eagle Medium (DMEM, Gibco-BRL) control media with 10% FBS (Gibco-BRL) and 1% antibiotic-antimycotic (Gibco-BRL) was added to neutralize collagenase activation, then centrifuged 1500 rpm (260 g) for 5 min. Cell pellets were resuspended with phosphate-buffered saline with 2% foetal bovine serum. The number of SVF cells was counted using the FACSCalibur cytometer (Becton Dickinson). 50,000 SVF cells were diluted with distilled water 0.5, transferred to an Eppendorf tube for the final SVF product, and stored at 4 °C before use [21,22].
2.3. PRP and SVF combination preparation
In the combination group of PRP and SVFs, 50,000 cells were added with 0.5 cc of PRP, so that the final product of PRP + SVFs was the same for both the injection treatment group and the topical group [22].
2.4. Vaseline
Vaseline, also known as white petrolatum, acts as an additive to make a product [23]. Vaseline is often used for hair oils, body lotions, ointments, and topical creams. Vaseline was used in this study because it has the ability to become a wound moisturizer (moist) on a wound site [24].
2.5. Deep dermal burn model
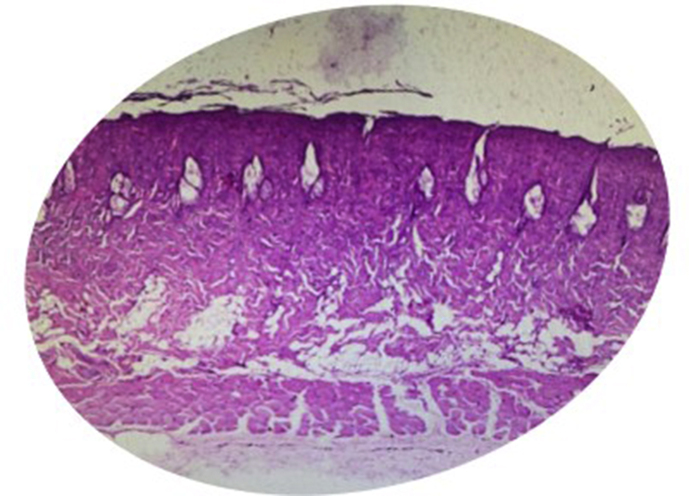
Deep dermal burn models were prepared following the method described by Guo et al. [25] with modifications. After an adaptation period of 1 week, rats were anesthetized by inhalation using ether in a special box until the state of consciousness decreased. The affected area was disinfected with Povidone-iodine 1%. After that, aluminium metal with a diameter of 10 mm was heated in hot water at 100 °C for 5 min. The hot metal was affixed for 3 s to the back of the rat. The pressure applied to the skin corresponded to a mass of 51 gr of aluminium metal to induce burns. This method obtained deep dermal burns which were confirmed by the results of histopathological examination (Fig. 1) [26]. Immediately after the procedure, analgesia with metamizole 50 mg/kg and amoxicillin 15 mg/kg antibiotics were given orally [25,26].
Fig. 1.
Histopatological deep dermal burn model in our study.
2.6. Wound analysis
Skin tissue was subjected to histopathological analysis to assess deep dermal burns.
2.7. Combination stem cell and vaseline implantation
There was one negative control group and three treatment groups. Group A was the control of TGF levels in healthy rats without treatment. In Group B, wound care was performed using standard moist (Vaseline) which was applied to the wound surface. In Group C, a topical combination of PRP + SVFs was administered in gel form and placed on the wound surface, each with a total volume of 0.5 cc of PRP +50,000 SVFs cells per experimental animal. In Group D, subcutaneous injections of PRP and SVFs were administered to the four edges of the wound at 12, 3, 6, 9, and the center of the wound 0.1 cc each with a total volume of 0.5 cc of PRP +50,000 SVFs cells per experimental animal. The rats were then fed sufficiently and given oral analgesics and antibiotics.
2.8. TGF beta test
TGF beta examination was carried out 6 times, on day 0 (24 h) before the burn treatment, then blood was taken 1, 4, 7, 10, and 14 days after treatment. All blood samples were examined by TGF beta Sandwich ELISA with Catalogue No. MBS175833 which was purchased from MyBioSource, Inc [[33], [34]].
2.9. Statistical analysis
All data are presented as mean ± SD. Data were analysed using the Levene and independent t-tests using SPSS software for Windows 21 (IBM SPSS Statistics for Windows, Version 21.0. IBM Corp., Armonk, NY). A p-value less than 0.05 was considered statistically significant.
3. Results
In this study, as many as 64 rats were divided into 4 treatment groups: Group A (negative control), Group B (topical Vaseline and burn wound), Group C (Topical PRP and SVFs and burn wound), and Group D (PRP Injection and SVF and burn wound).
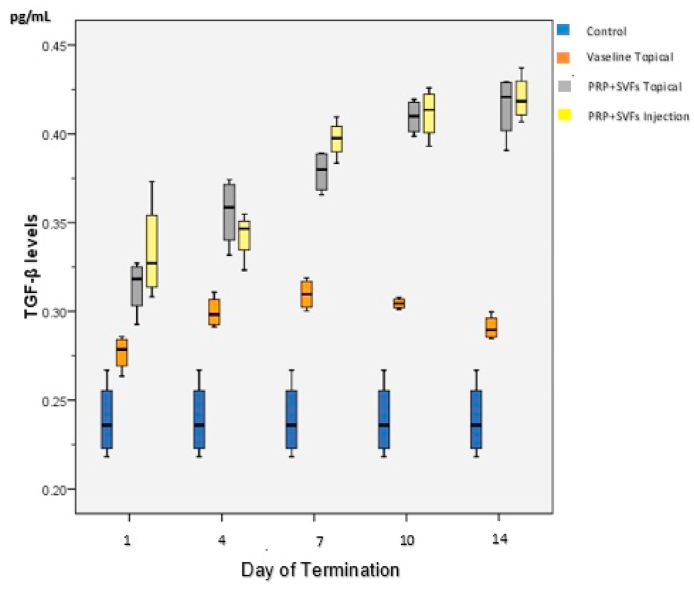
As shown in Table 1, the highest TGF-β levels were found in Group B (topical Vaseline and burns) which increased slowly starting the first day (0.267) and reached its peak on the 7th day (0.309), then slowly decreased until the 7th and 14th days (0.290). In Group C (topical PRP and SVFs and burns), levels of TGF-β started to increase slowly from the first day (0.314) and continued to increase until the 14th day (0.415). TGF-β levels in Group D (PRP injection and SVF and burns) began to increase on the first day of injection (0.333), and continued to increase until the 14th day (0.402) (Fig. 2).
Table 1.
The average change in TGF-β levels in each treatment group.
| Treatment Group (n = 16) | Average level of TGF-β on termination day (Mean ± SD) (pg/mL) |
||||
|---|---|---|---|---|---|
| 1 | 4 | 7 | 10 | 14 | |
| A | 0.239 ± 0.021 | ||||
| B | 0.276 ± 0.009 | 0.299 ± 0.008 | 0.309 ± 0.008 | 0.304 ± 0.002 | 0.290 ± 0.006 |
| C | 0.314 ± 0.015 | 0.355 ± 0.019 | 0.378 ± 0.011 | 0.409 ± 0.009 | 0.415 ± 0.018 |
| D | 0.333 ± 0.028 | 0.342 ± 0.013 | 0.397 ± 0.010 | 0.411 ± 0.014 | 0.420 ± 0.012 |
Fig. 2.
Average changes in TGF-β levels in each treatment group.
3.1. Comparison of Group A (negative control) and Group B (topical vaseline)
As shown in Table 2, the TGF-β level after giving Vaseline on the first to the 14th day had a p-value <0.05. Therefore, there was a significant difference from the TGF-β level in the control group. These results show that H0 is rejected and H1 is accepted. There was a significant effect on the TGF-β levels of deep dermal burns after giving Vaseline on days 1, -4, -7, -10, and −14.
Table 2.
Comparison of TGF-β levels between Groups A and B.
| Day of Termination | Group A (mean ± SD) (pg/mL) |
Control B (mean ± SD) (pg/mL) |
*p value | **p value |
|---|---|---|---|---|
| 1 | 0.276 ± 0.009 | 0.168 | 0.019 | |
| 4 | 0.299 ± 0.008 | 0.139 | 0.002 | |
| 7 | 0.239 ± 0.021 | 0.309 ± 0.008 | 0.134 | 0.001 |
| 10 | 0.304 ± 0.002 | 0.035 | 0.008 | |
| 14 | 0.290 ± 0.006 | 0.085 | 0.004 |
*Levene test **Independent t-test.
3.2. Comparison of Group B (vaseline) and Group C (Topical SVFs and PRP)
As shown in Table 3, the ratio of TGF-β levels after administering topical stem cells and Vaseline on the first to the 14th days has a value of p < 0.05, which represents a significant difference in TGF-β levels between the two groups. These results show that H0 is rejected and H1 is accepted; that is, topical stem cell administration performed better than Vaseline for changes in TGF-β levels in deep dermal burns on days 1, -4, -7, -10, and - 14.
Table 3.
Comparison of TGF-β levels between Groups B and C.
| Day of Termination | Group B (mean ± SD) (pg/mL) |
Group C (mean ± SD) (pg/mL) |
*p value | **p value |
|---|---|---|---|---|
| 1 | 0.276 ± 0.009 | 0.314 ± 0.015 | 0.507 | 0.006 |
| 4 | 0.299 ± 0.008 | 0.355 ± 0.019 | 0.080 | 0.002 |
| 7 | 0.309 ± 0.008 | 0.378 ± 0.011 | 0.134 | <0.001 |
| 10 | 0.304 ± 0.002 | 0.409 ± 0.009 | 0.005 | <0.001 |
| 14 | 0.290 ± 0.006 | 0.415 ± 0.018 | 0.133 | <0.001 |
*Levene test ** Independent t-test.
3.3. Comparison of Group B (vaseline) and Group D (injection of SVFs and PRP)
As shown in Table 4, the ratio of TGF-β levels after giving stem cell injection and Vaseline on the first day to the 14th day has a p value < 0.05, which represents a significant difference in TGF-β levels between the two groups. Based on these results, H0 is rejected and H1 is accepted; that is, stem cell injection is better than Vaseline for changes in TGF-β levels in deep dermal burns on days 1, -4, -7, -10, and −14.
Table 4.
Comparison of TGF-β levels between Groups B and D.
| Day of Termination | Group B (mean ± SD) (pg/mL) |
Group D (mean ± SD) (pg/mL) |
*p value | **p value |
|---|---|---|---|---|
| 1 | 0.276 ± 0.009 | 0.333 ± 0.028 | 0.178 | 0.009 |
| 4 | 0.299 ± 0.008 | 0.342 ± 0.013 | 0.539 | 0.002 |
| 7 | 0.309 ± 0.008 | 0.397 ± 0.010 | 0.984 | <0.001 |
| 10 | 0.304 ± 0.002 | 0.411 ± 0.014 | 0.049 | <0.001 |
| 14 | 0.290 ± 0.006 | 0.420 ± 0.012 | 0.305 | <0.001 |
*Levene test ** Independent t-test.
3.4. Comparison of Group C (Topical SVFs and PRP) and Group D (SVFs and PRP injection)
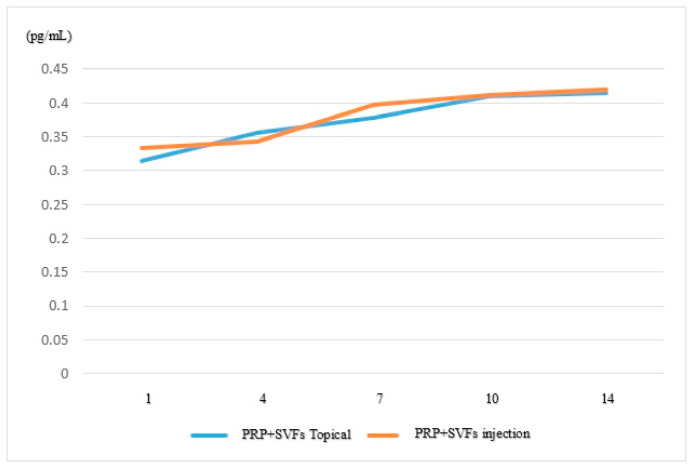
As shown in Table 5 and Fig. 3, the ratio of TGF-β levels after giving stem cell and topical stem cell injections on the first day to the 14th day has a value of p > 0.05. Therefore, the difference in TGF-β levels is not significant between the two groups. Based on these results, H1 is rejected and H0 is accepted; that is, the administration of stem cell injections is no better than giving topical stem cells to changes in levels of TGF-β deep dermal burns on days 1, -4, -7, -10, and −14.
Table 5.
Comparison of TGF-β levels between Groups C and D.
| Day of Termination | Group C (mean ± SD) (pg/mL) |
Group D (mean ± SD) (pg/mL) |
*p value | *p value |
|---|---|---|---|---|
| 1 | 0.314 ± 0.015 | 0.333 ± 0.028 | 0.349 | 0.266 |
| 4 | 0.355 ± 0.019 | 0.342 ± 0.013 | 0.309 | 0.316 |
| 7 | 0.378 ± 0.011 | 0.397 ± 0.010 | 0.437 | 0.061 |
| 10 | 0.409 ± 0.009 | 0.411 ± 0.014 | 0.507 | 0.825 |
| 14 | 0.415 ± 0.018 | 0.420 ± 0.012 | 0.499 | 0.679 |
*Levene test ** Independent t-test.
Fig. 3.
Comparison of TGF-β levels between Groups C and D.
4. Discussion
The TGF-β level of the Vaseline group had a significant difference compared to the control group; levels peaked on the 7th day of Vaseline administration. A study comparing a group given Vaseline to a control group with fibroblast parameters found that the 7th, 14th, and 21st days of Vaseline administration were better than the control group [27]. Zhang YS et al. found that TGF-β levels using Vaseline compared to a control group were higher on the 7th and 14th days; the TGF-β level in the control group was higher than the Vaseline β1 group [28]. Another study carried out for 35 days comparing pelnac, Vaseline, and control groups to TGF-β found that use of Vaseline was better than the pelnac and control groups with significant results (p < 0.001) [29].
This study found that the administration of PRP + SVFs topically or by injection was significantly better at increasing the expression of TGF-β up to the 14th day than the administration of Vaseline or controls (p < 0.05). PRP contains many growth factors (such as TGF-β, PDGF, and VEGF) that mediate angiogenesis. These growth factors stimulate cell proliferation, migration, and capillary tube formation via multiple signalling pathways, such as extracellular regulated kinase (ERK) and calcineurin (CaN)/nuclear factor from the activated T-cell signalling pathway (NFAT), whereas SVFs contain many cell types such as ADSCs, pre-adipocytes, fibroblasts, endothelial cells, macrophages, and lymphocytes [22]. ADSCs have been reported to be composed of many cytokines and growth factors that can influence parameters such as angiogenesis, fibroblasts, mononuclear infiltrates, and collagen production. Expression of potent angiogenic agents such as vascular endothelial growth factor (VEGF), fibroblast migration, and stimulators of activity such as transforming growth factor (TGF) and macrophage chemotaxis are expressed by ADSC [22,31]. Collectively, these processes can accelerate the proliferative phase and build more favorable conditions for wound repair as observed in our results.
In our study, the results showed that there was no significant difference between the administration of PRP + SVFs by injection or topically. The administration of PRP + SVFs by injection was not better at increasing the expression of TGF-β than by topical administration of PRP + SVFs. In a previous study involving 24 patients with chronic lower limb ulcers, wounds were injected with PRP every 2 weeks, and successful wound closure and reepithelialisation were obtained in 20 wounds. In addition, the use of autologous PRP gel in treating 59 patients with acute soft tissue wounds of the extremities showed that the administration of PRP gel was valuable and effective in the management of acute trauma wounds [5].
Wound healing of the skin involves the regulation of inflammation, cell proliferation, remodelling, extracellular matrix deposition, angiogenesis, and epithelialization. PRP combined with adipose-derived stem cells—in this case SVFs—can improve the healing of the skin layer in mice by reducing infiltration of the inflammatory process, increasing collagen deposition, promoting angiogenesis and neurogenesis [29]. The combination of adipose-derived stem cells and PRP is effective in increasing growth factor concentrations (IGF-1, TGF- β 1, HGF, and VEGF) [22].
The most important growth factors in PRP are TGF-β and PDGF. Both affect each stage of wound healing by stimulating cell growth. TGF-β regulates cell differentiation, proliferation, chemotaxis, and synthesis of several extracellular matrix proteins. The level of TGF-β was four times higher in PRP than in PPP (platelet-poor plasma). Furthermore, TGF-β further enhances superior basal cell proliferation and epidermal regeneration. Collagen, fibronectin, and glycosaminoglycan synthesis from fibroblasts are stimulated by TGF-β. TGF-β triggers collagen synthesis and accelerates collagen maturation in the initial period of wound healing [32]. Previous studies have described a role for TGF-β in the formation of hypertrophic scars. TGF-β may be a predictive factor in the formation of burn scars with hypertrophic scars. The study explained that burns that heal well and in the absence of hypertrophic scarring show the formation of TGF-β in plasma for 2 weeks after the burn. However, burns with hypertrophic scarring did not show an initial increase in TGF-β levels [17].
Many studies, including ours, support the superiority of the combination of SVF and PRP compared to Vaseline or control groups for wound healing and tissue regeneration. Given the limitations of conventional approaches for wound healing, including skin grafts, our findings have important practical implications and address a clinical issue. Furthermore, our results may be of general interest to researchers interested in additive and synergistic effects of therapeutic strategies involving stem cells.
Monitoring TGF-β levels in this study was only carried out for 14 days (proliferation phase). It is better to monitor TGF-β levels until the remodelling phase, so that researchers can determine whether the appropriate therapy has been given in burn cases. This study only recorded and evaluated TGF-β levels in the four treatment groups and did not assess other outcomes in rats, such as clinical appearance, wound area, and so on, which supports the assessment of the effectiveness of a therapy. We recommend that further research be carried out by including these variables.
However, further preclinical studies were conducted on the effect of administering a combination of SVFs and PRP on growth factor levels, especially TGF-β in the healing process of deep dermal burns by assessing the quantity of burn healing through histopathology.
5. Conclusion
From this study, it can be concluded that the administration of the combination of PRP + SVFs by injection and topically in rats induced by deep dermal burns can increase TGF-β levels significantly compared to the group with Vaseline administration.
Funding
No funding or sponsorship.
Registration of research studies
None.
Ethical approval
All procedure for Animal experiment has been approved by Ethics Commission Faculty of Medicine, Hasanuddin University Number: 1029/UN4.6.4.5.31/PP36/2019.
Consent
This manuscript does not involve human participants, human data, or human tissue.
Author contribution
SRL, FJ, FRC, MF, ASP, and BS wrote the manuscript and participated in the study design. SRL, FJ, FRC, WS, ASB, MNM, and AAI drafted and revised the manuscript. SRL, FJ, FRC, MF, ASP, and BS performed deep dermal burn models, stem cell preparation and implantation. SRL, FRC, MF, ASP, and BS performed bioinformatics analyses and revised the manuscript. All authors read and approved the final manuscript.
Guarantor
Sachraswaty R. Laidding.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Acknowledgment
A higher appreciation to all staff from the Hasanuddin University Medical Research Center (HUMRC), Makassar, Indonesia.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.amsu.2020.11.088.
Contributor Information
Sachraswaty Rachman Laidding, Email: sachras1276@gmail.com.
Fonny Josh, Email: fonnyjosh2003@yahoo.com.
Francisca, Email: ikaduma987@gmail.com.
Muhammad Faruk, Email: faroex8283@gmail.com.
Andi Sinapati Palissei, Email: andhisinapatipalissei@gmail.com.
Bayu Satria, Email: drbayusatria@gmail.com.
Warsinggih, Email: kbd.warsinggih@gmail.com.
Agussalim Bukhari, Email: agussalimbukhari@yahoo.com.
Muh Nassrum Massi, Email: nasrum2000@yahoo.com.
Andi Asadul Islam, Email: andiasadul@yahoo.com.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Demling R.H. The burn edema process: current concepts. J. Burn Care Rehabil. 2005;26:207–227. [PubMed] [Google Scholar]
- 2.Stokes M.A.R., Johnson W.D. Burns in the third world: an unmet need. Ann. Burns Fire Dis. 2017;30:243–246. https://pubmed.ncbi.nlm.nih.gov/29983673 [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Beekman J., Hew J., Jackson S., Issler-Fisher A.C., Parungao R. Elsevier B.V.; 2018. Burn Injury: Challenges and Advances in Burn Wound Healing, Infection, Pain and Scarring. [DOI] [PubMed] [Google Scholar]
- 4.Benson A., Dickson W.A., Boyce D.E. ABC of wound healing: Burns. BMJ. 2006;333:609324. doi: 10.1136/sbmj.0609324. [DOI] [Google Scholar]
- 5.Gentile P., Scioli M.G., Bielli A., Orlandi A., Cervelli V. Concise review: the use of adipose-derived stromal vascular fraction cells and platelet rich plasma in regenerative plastic surgery. Stem Cell. 2017;35:117–134. doi: 10.1002/stem.2498. [DOI] [PubMed] [Google Scholar]
- 6.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So. Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J., Minn K.W., Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch. Plast. Surg. 2012;39:585–592. doi: 10.5999/aps.2012.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraro G.A., Mizuno H., Pallua N. Adipose stem cells: from bench to bedside. Stem Cell. Int. 2016;2016:6484038. doi: 10.1155/2016/6484038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raposio E., Bertozzi N., Bonomini S., Bernuzzi G., Formentini A., Grignaffini E. Adipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapy., wounds a compend. Clin. Res. Pract. 2016;28:126–131. [PubMed] [Google Scholar]
- 10.Borrione P., Di Gianfrancesco A., Pereira M.T., Pigozzi F. Platelet-rich plasma in muscle healing. Am. J. Phys. Med. Rehabil. 2010;89:854–861. doi: 10.1097/PHM.0b013e3181f1c1c7. [DOI] [PubMed] [Google Scholar]
- 11.Duran A., Yasar S., Gunes P., Aytekin S., Duran A. 2016. Clinical and Histopathological Evaluation of the Effects of Platelet Rich Plasma, Platelet Poor Plasma and Topical Serum Physiologic Treatment on Wound Healing Caused by Radiofrequency Electrosurgery in Rats. [Google Scholar]
- 12.Stessuk T., Puzzi M.B., Chaim E.A., Alves P.C.M., de Paula E.V., Forte A. Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells: stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro. Arch. Dermatol. Res. 2016;308:511–520. doi: 10.1007/s00403-016-1676-1. [DOI] [PubMed] [Google Scholar]
- 13.Tohidnezhad M., Varoga D., Wruck C.J., Brandenburg L.O., Seekamp A., Shakibaei M. Platelet-released growth factors can accelerate tenocyte proliferation and activate the anti-oxidant response element. Histochem. Cell Biol. 2011;135:453–460. doi: 10.1007/s00418-011-0808-0. [DOI] [PubMed] [Google Scholar]
- 14.Martins R.P., Hartmann D.D., de Moraes J.P., Soares F.A.A., Puntel G.O. Platelet-rich plasma reduces the oxidative damage determined by a skeletal muscle contusion in rats. Platelets. 2016;27:784–790. doi: 10.1080/09537104.2016.1184752. [DOI] [PubMed] [Google Scholar]
- 15.Yun Y.-R., Won J.E., Jeon E., Lee S., Kang W., Jo H. Fibroblast growth factors: biology, function, and application for tissue regeneration. J. Tissue Eng. 2010;2010:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chieregato K., Castegnaro S., Madeo D., Astori G., Pegoraro M., Rodeghiero F. Epidermal growth factor, basic fibroblast growth factor and platelet-derived growth factor-bb can substitute for fetal bovine serum and compete with human platelet-rich plasma in the ex vivo expansion of mesenchymal stromal cells derived from adipose tiss. Cytotherapy. 2011;13:933–943. doi: 10.3109/14653249.2011.583232. [DOI] [PubMed] [Google Scholar]
- 17.Penn J.W., Grobbelaar A.O., Rolfe K.J. The role of the TGF-β family in wound healing, burns and scarring: a review. Int. J. Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Grant M.B., Khaw P.T., Schultz G.S., Adams J.L., Shimizu R.W. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest. Ophthalmol. Vis. Sci. 1992;33:3292–3301. [PubMed] [Google Scholar]
- 19.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.1136/bmj.c332. c332–c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josh F., Kobe K., Tobita M., Tanaka R., Suzuki K., Ono K. Accelerated and safe proliferation of human adipose-derived stem cells in medium supplemented with human serum. J. Nippon Med. Sch. 2012;79:444–452. doi: 10.1272/jnms.79.444. [DOI] [PubMed] [Google Scholar]
- 22.Tajima S., Tobita M., Orbay H., Hyakusoku H., Mizuno H. Direct and indirect effects of a combination of adipose-derived stem cells and platelet-rich plasma on bone regeneration. Tissue Eng. 2014;21 doi: 10.1089/ten.TEA.2014.0336. [DOI] [PubMed] [Google Scholar]
- 23.Petry T., Bury D., Fautz R., Hauser M., Huber B., Markowetz A. Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicol. Lett. 2017;280:70–78. doi: 10.1016/j.toxlet.2017.07.899. [DOI] [PubMed] [Google Scholar]
- 24.Sethi A., Kaur T., Malhotra S.K., Gambhir M.L. Moisturizers: the slippery road. Indian J. Dermatol. 2016;61:279–287. doi: 10.4103/0019-5154.182427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H.-F., Ali R.M., Hamid R.A., Zaini A.A., Khaza’ai H. A new model for studying deep partial-thickness burns in rats. Int. J. Burns Trauma. 2017;7:107–114. [PMC free article] [PubMed] [Google Scholar]
- 26.Tavares Pereira D.dos S., Lima-Ribeiro M.H.M., de Pontes-Filho N.T., Carneiro-Leão A.M.dos A., Correia M.T.dos S. Development of animal model for studying deep second-degree thermal burns. J. Biomed. Biotechnol. 2012;2012:460841. doi: 10.1155/2012/460841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaghardoost R., Mousavi Majd S.G., Tebyanian H., Babavalian H., Malaei L., Niazi M. The healing effect of sesame oil, camphor and honey on second degree burn wounds in rat. World J. Plast. Surg. 2018;7:67–71. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., He J., Xiao G., Li Q. [Effect of platelet-rich plasma on the proliferation and adipogenic differentiation of human adipose-derived stem cells in vitro] Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:525–528. [PubMed] [Google Scholar]
- 29.Liu T., Qiu C., Ben C., Li H., Zhu S. One-step approach for full-thickness skin defect reconstruction in rats using minced split-thickness skin grafts with Pelnac overlay, Burn. Trauma. 2019;7 doi: 10.1186/s41038-019-0157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoso A.L., Bachion M.M., Morais J. de M., Fantinati M.S., de Almeida V.L.L., Lino Júnior R.S. Adipose tissue stromal vascular fraction in the treatment of full thickness burns in rats. Acta Cir. Bras. 2016;31:578–585. doi: 10.1590/S0102-865020160090000002. [DOI] [PubMed] [Google Scholar]
- 32.Ozcelik U., Ekici Y., Bircan H.Y., Aydogan C., Turkoglu S., Ozen O. Effect of topical platelet-rich plasma on burn healing after partial-thickness burn injury. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2016;22:1903–1909. doi: 10.12659/MSM.895395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasution R.A., Islam A.A., Hatta M., Prihantono, Turchan A., Nasrullah, Faruk M. Role of CAPE in reducing oxidative stress in animal models with traumatic brain injury. Ann. Med. Surg. 2020;57:118–122. doi: 10.1016/j.amsu.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warsinggih, Irawan B., Labeda I., Lusikooy R.E., Sampetoding S., Kusuma M.I., Uwuratuw J.A., Syarifuddin E., Prihantono, Faruk M. Association of superoxide dismutase enzyme with staging and grade of differentiation colorectal cancer: a cross-sectional study. Ann. Med. Surg. 2020;58:194–199. doi: 10.1016/j.amsu.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.