Figure 4.
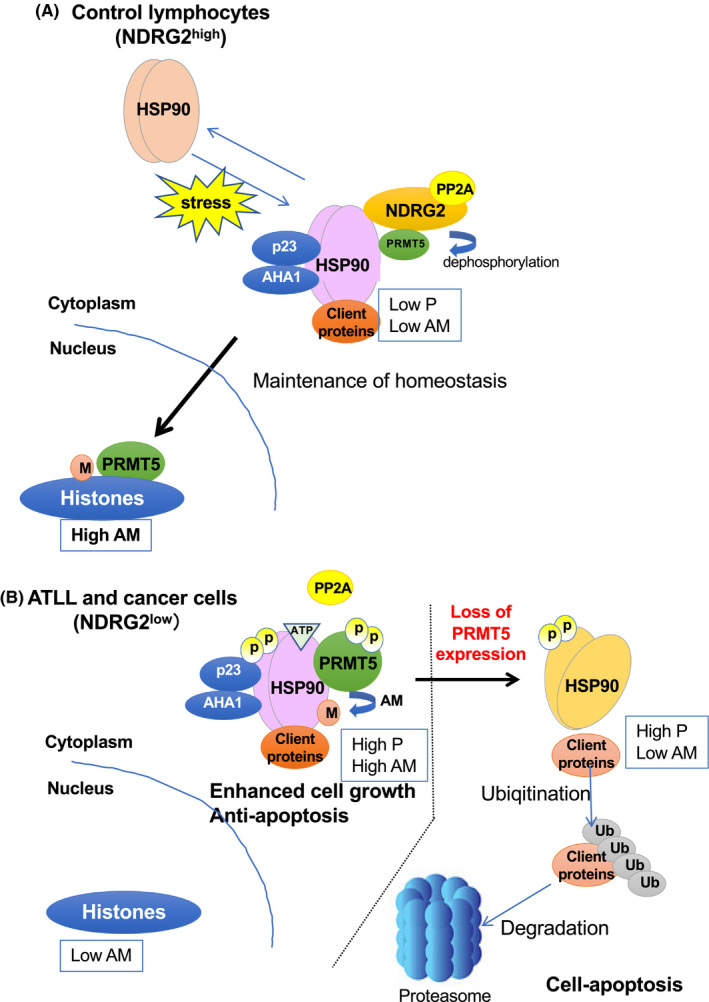
Regulation of heat shock protein 90 (HSP90A) function by arginine methylation of protein arginine methyltransferase 5 (PRMT5) via the N‐myc downstream‐regulated gene 2 (NDRG2)/protein phosphatase 2A (PP2A) complex (NDRG2/PP2A). Heat shock protein (HSP) 90, an ATP‐dependent molecular chaperone, is essential for oncogenic transformation through supporting a wide variety of activated oncoproteins, kinases, transcription factors and others. Although the activity of HSP90 is regulated by many protein modifications such as phosphorylation and acetylation, we first reported a mechanism for the activity regulation of HSP90 by arginine methylation through PRMT5. Moreover, the NDRG2/PP2A complex regulates the arginine methyltransferase activity of PRMT5 by its phosphorylation status. (A) In normal cells, the NDRG2/PP2A complex dephosphorylates PRMT5 and unphosphorylated PRMT5 is mainly localized to the nucleus and leads to arginine methylation of histones; however, cytoplasmic PRMT5 cannot catalyze the arginine residues in HSP90. 16 (B) In adult T‐cell leukemia/lymphoma (ATLL) or other cancer cells with low expression of NDRG2, PRMT5 is mainly localized in the cytoplasm and is highly phosphorylated by the downregulation of NDRG2, and induces the methylation of HSP90A arginine residues, leading to the enhancement of its chaperone activity (AHA1 and p23) and stabilization of client proteins, including AKT, NF‐κB essential modulator (NEMO) or others, contributing to enhanced cell growth and anti‐apoptosis. In the right half of the figure, knocking down PRMT5 expression in NDRG2‐deficient tumors induces cell apoptosis via the reduction of HSP90A function and the degradation of client proteins via activation of the ubiquitin‐proteasome pathway, suggesting that enzymatic inhibition of PRMT5 is a potential therapeutic target because it causes synthetic lethality in NDRG2‐deficient cancer or ATLL cells. 16 This figure has been adapted from reference 16 with permission
