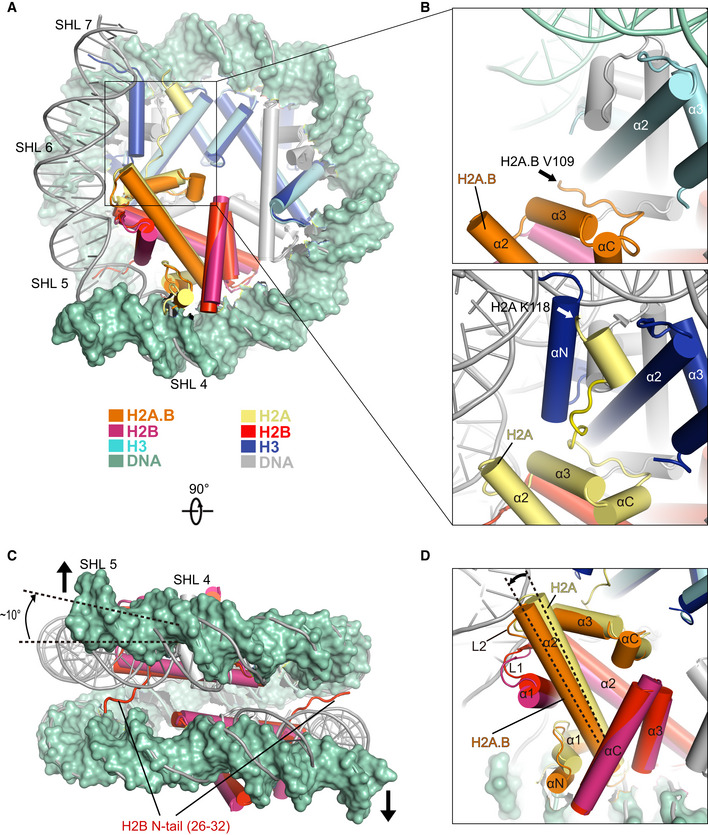
Comparison of the cryo‐EM structure of H2A.B‐NCP with the structure of canonical nucleosome (PDB: 3LZ0) in disc view. Histone octamers containing H2A.B and canonical H2A were superimposed for comparison. The ˜ 103 bp DNA of H2A.B‐NCP in green color is shown in surface mode. The 145 bp DNA of canonical nucleosome is colored in gray. Histones H2A.B, H2B, H3, and DNA at SHL 5 through SHL 7 are highlighted to show the structural differences between two nucleosomes. Histones H2A.B, H2B, and H3 in H2A.B‐NCP are colored in orange, warmpink, and cyan. Their counterparts in canonical nucleosome are colored in yellow, red, and blue. Histone H4 is colored in gray.
Close‐up view of the structures of H2A.B C‐terminal regions (top) and H2A C‐terminal regions (bottom). Black and white arrows indicate the last residue at the C‐terminus of H2A.B (V109) and H2A (K118) that are observable in the structures.
Comparison of the cryo‐EM structure of H2A.B‐NCP with the structure of canonical nucleosome in gyre view. Arrows indicate the direction of H2A.B‐NCP DNA movement during nucleosome gaping transition. The dashed lines indicate the ˜ 10 degrees rotation of H2A.B DNA at SHL 5. H2B N‐tail refers to H2B residues 26–32 which are exclusively observed in the canonical nucleosome structure. Histones H2B in H2A.B‐NCP are colored in warmpink, Their counterparts in canonical nucleosome are colored in red. H2A.B‐NCP DNA in green is shown in surface mode for clear comparison.
Close‐up view of H2A.B‐H2B dimer in the structural comparison. The secondary structure of H2A.B‐H2B dimer is indicated. The dashed lines highlight the tilt of H2A.B α2‐helix.