Abstract
Background:
Clinical studies have shown that palbociclib improves progression-free survival in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2−) patients with advanced breast cancer (ABC). However, there are insufficient data on its use in a real-world setting in Japan. The aim of this study was to investigate the effectiveness, predictive factors, and safety of palbociclib among Japanese patients in routine clinical practice.
Methods:
Between December 1, 2017, and April 30, 2019, we recruited patients from 9 hospitals and retrospectively evaluated the data on HR+/HER2− patients with ABC who received palbociclib for at least 1 week. The correlation between time-to-treatment discontinuation (TTD) and clinical background was investigated via univariate and multivariate analyses using Cox hazards models.
Results:
A total of 177 women were available for analysis. Of these patients, 58 (33%) patients were treated with palbociclib with an aromatase inhibitor and 117 (66%) patients were treated with palbociclib and a selective estrogen receptor degrader. Approximately three-fourths of the patients (n = 130, 73%) received palbociclib as third- or later-line therapy. One-third of the patients had 3 or more metastatic sites (n = 59, 33%), and one-third of the patients had liver metastasis (n = 59, 33%). The median follow-up duration at the time of data cutoff was 8.9 months, the median TTD was 6.3 months, and the median overall survival was not reached. Liver metastasis (hazard ratio [HR]: 1.54 [95% confidence interval {CI}: 1.03-2.27]), high serum lactate dehydrogenase (LDH) level (>300 U/L) (HR: 2.58 [95% CI: 1.49-4.26]), and high neutrophil-to-lymphocyte ratio (NLR) (⩾3.0) (HR: 1.76 [95% CI: 1.13-2.69]) were significantly associated with shorter TTD. The most common hematologic adverse event was neutropenia, which occurred in 93% of the patients.
Conclusion:
Based on the results of the pivotal phase 3 trials, the median TTD recorded in this study was shorter than expected. Our results suggest that liver metastasis, serum LDH level, and NLR may be predictive factors for HR+/HER2− ABC treatment outcomes.
Keywords: Advanced breast cancer, lactate dehydrogenase, neutrophil-to-lymphocyte ratio, palbociclib
Introduction
The most common subtype of early breast cancer is the hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2−) type. Patients with advanced breast cancer (ABC) are also mostly diagnosed with the HR+/HER2− type, and they are initially treated with endocrine therapy (ET) if the disease is not life-threatening. Palbociclib is a cyclin-dependent kinase (CDK) 4/6 inhibitor, which became available for the treatment of patients with ABC in Japan on December 1, 2017. This drug is a highly selective CDK4/6 inhibitor. Preclinical studies have shown that palbociclib arrests cell-cycle progression, leading to suppression of tumor cell proliferation, especially in HR+ breast cancer cells.1,2 Several clinical trials have shown that a combination treatment with palbociclib and ET significantly prolongs progression-free survival (PFS) compared with treatment with Et alone.3-5 Several studies have also demonstrated the efficacy of this combination treatment among Asian patients, including Japanese patients.6-8
Previous translational studies have evaluated circulating tumor DNA and cyclin E1 messenger RNA (mRNA) gene expression to identify a predictive biomarker of palbociclib.9,10 However, these data are not yet available for use in clinical practice. In addition, most patients have different backgrounds and characteristics, such as age, line of therapy, and comorbidities, from the subjects in these clinical studies. Given that previous Japanese studies have only investigated treatment with palbociclib in clinical trial settings or small populations,11-13 it is essential to evaluate the effectiveness of palbociclib in a larger, real-world setting and investigate the predictive factors for its efficacy using lactate dehydrogenase (LDH)14 and neutrophil-to-lymphocyte ratio (NLR)15 as clinical markers. In this study, we investigated the effectiveness, predictive factors, and safety of palbociclib for the treatment of patients with ABC in routine clinical practice.
Patients and Methods
Patients and study design
This was a multicenter, retrospective, observational cohort study. Between December 1, 2017, and April 30, 2019, consecutive patients with ABC were recruited from 9 hospitals under the Kobe Breast Cancer Oncology Group (study registration no.: UMIN 000036224). We only included women who had HR+/HER2− ABC and who were treated with palbociclib for at least 1 week. There were no exclusion criteria regarding menopausal status, line of therapy, Eastern Cooperative Oncology Group performance status (ECOG-PS), and use of combination drugs. Data were collected from medical records, laboratory data, and prescription orders at each facility.
We obtained ethical approval from the local institutional review board of each participating institution. This study collected only retrospective clinical data and presents no risk to the anonymity of the participants. Therefore, the institutional review board of each institution waived the requirement for informed consent.
Data collection
Both HR and HER2 statuses were defined based on the guidelines of the American Society of Clinical Oncology/College of American Pathologists16,17 and categorized as either primary or metastatic tumors. We classified tumors as metastatic if they spread to the lung, liver, bone, brain, distant lymph node, or other organs or tissues. The number and location of organs that were sites of metastasis were summarized regardless of whether the metastasis was single or multiple. Other metastatic involvements such as peritoneal metastasis and lymphangitic carcinomatosis were included as well. Treatment history for ABC was recorded as the number of chemotherapies (CTs) or ETs (including administration of everolimus) the patient underwent. We collected baseline peripheral blood data on LDH levels and NLR before the first cycle of palbociclib treatment. NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. We defined high LDH as LDH higher than 300 U/L18 and high NLR as NLR that was 3.0 or higher.19 We calculated the total dose and relative dose intensity (RDI) and recorded the starting and ending doses of palbociclib.
Outcomes
The study outcomes were as follows: (1) time-to-treatment discontinuation (TTD), which was defined as the time from the start of treatment with palbociclib until the time of discontinuation due to any cause; (2) overall survival (OS), which was defined as the duration between the initiation of treatment with palbociclib and death of any cause; and (3) safety of palbociclib. Only safety data regarding hematologic adverse events (AEs) were collected because it is difficult to correctly retrieve data regarding nonhematologic AEs from an electronic medical record. Information about the occurrence of hematologic AEs retrieved from laboratory data was recorded according to the Common Terminology Criteria for Adverse Events version 5.0. The correlation between TTD and patient characteristics, including LDH and NLR, was also analyzed to investigate the clinical predictive markers of palbociclib.
Statistical analysis
Descriptive statistics were used to analyze demographic and clinical characteristics. To assess TTD, we used the Kaplan-Meier method and the log-rank test. We defined significance as P < .05 and performed a forward-backward stepwise regression; enter limit and remove limit were P = .1 and P = .11, respectively. Univariate and multivariate analyses were performed to estimate hazard ratios using Cox proportional hazard regression. We set the RDI cutoff at 80% and 60% based on the recommended dose reduction of palbociclib (100 mg and 75 mg, respectively). All statistical analyses were performed using the JMP 12 statistical program (JMP 12.0.0; SAS Institute, Cary, NC, USA).
Results
Patients
We recruited a total of 179 patients; however, only 177 were available for analysis. Two patients were ineligible because they had unknown medical histories. The patient characteristics are outlined in Table 1. The median age of the patients was 65 years, and more than 90% of the patients had ECOG-PS ⩽1. Premenopausal/perimenopausal patients received gonadotropin-releasing hormone agonists. The progesterone receptor statuses of 2 patients were unknown. All, except 3 patients, had metastatic disease; 33% of the patients had more than 3 sites of metastases. One-third of the patients and 50% of the patients who were treated with palbociclib as a first-line therapy had liver metastasis (this result is not shown). Approximately half of the patients underwent CT, and 29% received everolimus for advanced disease. The median line of therapy was the fourth, and the number of patients treated with palbociclib as the first, second, and third or later lines of therapy were 20, 27, and 130, respectively. The data on serum LDH level were available for 176 patients. The median serum LDH level was 212.5 U/L, and 11% of patients had high LDH levels (>300 U/L). Neutrophil-to-lymphocyte ratio data were available for 165 patients, and the median NLR was 2.2. Patients who had high NLR (⩾3.0) accounted for 26% of the total population.
Table 1.
Patient characteristics.
| Characteristics | |
|---|---|
| Age, y | |
| Median (range) | 65 (36-87) |
| <70, n (%) | 124 (70) |
| Menopausal status, n (%) | |
| Post | 149 (84) |
| ECOG-PS, n (%) | |
| 0/1 | 166 (94) |
| Hormone receptor status, n (%)a | |
| ER+/PgR+ | 130 (74) |
| ER+/PgR− | 45 (26) |
| De novo metastatic disease, n (%) | 54 (31) |
| Recurrent, n (%) | 123 (69) |
| Number of metastatic sites, n (%) | |
| 0 | 3 (2) |
| 1-2 | 115 (65) |
| ⩾3 | 59 (33) |
| Bone-only metastasis, n (%) | 18 (10) |
| Liver metastasis, n (%) | 59 (33) |
| Treatment lines of palbociclib, median (range) | 4 (1-13) |
| First line, n (%) | 20 (11) |
| Second line, n (%) | 27 (15) |
| Third line or later, n (%) | 130 (73) |
| History of treatment for ABC, n (%) | |
| Chemotherapy | 97 (55) |
| Endocrine therapy | 153 (86) |
| Everolimus | 51 (29) |
| Treatment combination with palbociclib, n (%) | |
| AI | 58 (33) |
| SERD | 117 (66) |
| SERM | 2 (1) |
| Serum LDH, medianb | 212.5 |
| High LDH, n (%) | 19 (11) |
| Low LDH, n (%) | 157 (89) |
| NLR, medianc | 2.2 |
| High NLR, n (%) | 43 (26) |
| Low NLR, n (%) | 122 (74) |
Abbreviations: ABC, advanced breast cancer; AI, aromatase inhibitor; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; ER, estrogen receptor; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PgR, progesterone receptor; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulator.
PgR statuses of 2 patients were unknown.
Serum LDH level of 1 patient was unknown. High LDH indicates LDH level more than 300 U/L.
NLRs of 12 patients were unknown. High NLR indicates NLR more than 3.
Treatment outcomes
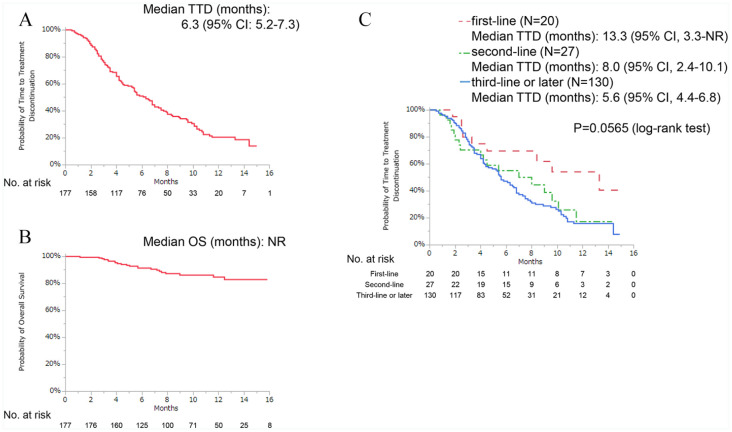
The date of data cutoff was April 30, 2019. The median follow-up duration was 8.9 months (range: 1.1-15.8 months). At the time of data cutoff, 56 patients were receiving treatment. The median TTD was 6.3 months (95% confidence interval [CI]: 5.2-7.3 months), and the median OS was not reached (Figure 1A and B). The median TTD was 13.3 months for the first-line therapy setting, followed by 8.0 and 5.6 months for the second-line therapy and third- or later-line therapy settings, respectively (Figure 1C).
Figure 1.
Kaplan-Meier curves of (A) TTD, (B) OS, and (C) TTD indicating first-, second-, and third- or later-line therapy with palbociclib.
CI indicates confidence interval; OS, overall survival; TTD, time-to-treatment discontinuation.
The reasons for permanent discontinuation of treatment were disease progression (n = 94), AE (n = 20), financial issues (n = 3), and others (n = 4).
Dose modification
The number of patients who received an initial palbociclib dose of 125 mg were 153 (86%). Of these patients, 107 (70%) patients required dose reduction to 100 mg, 75 mg or 50 mg. The remaining 24 (14%) patients received an initial palbociclib dose of 100 mg (n = 19, 11%) or 75 mg (n = 5, 3%). The final doses were 100 mg (n = 64, 36%), 75 mg (n = 60, 34%), 125 mg (n = 47, 27%), and 50 mg (n = 6, 3%). Dose escalation from 75 mg to 100 mg or 125 mg was observed in 2 cases.
Univariate and multivariate analysis
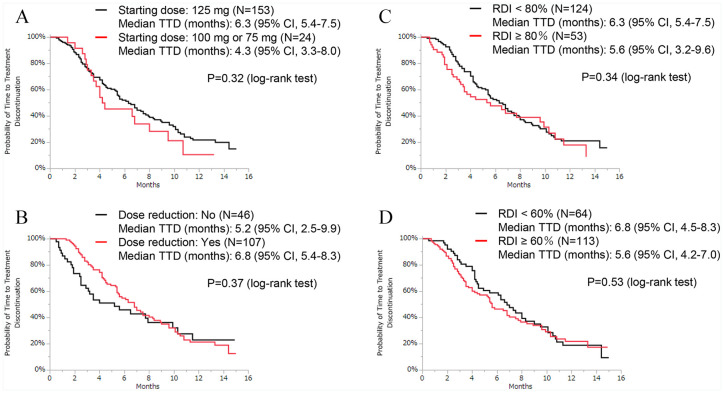
The results of the univariate analysis showed that first-line therapy, 3 or more metastatic sites, liver metastasis, and high LDH levels were significantly associated with TTD (Table 2). On the Kaplan-Meier curve, there was no significant difference between patients who received an initial dose of 125 mg and those who did not (Figure 2A). We defined patients in the dose reduction group as those who had their doses decreased to 100 mg, 75 mg or 50 mg from 125 mg. The TTD was not different between the dose reduction and no dose reduction groups (Figure 2B). The median RDI was 68%, and the TTD curve showed no significant differences when 80% or 60% was used as the RDI cutoff point (Figure 2C and D). In the multivariate analysis, the hazard ratios for first-line therapy, liver metastasis, high LDH, and high NLR were 0.36 (95% CI: 0.16-0.72), 1.54 (95% CI: 1.03-2.27), 2.58 (95% CI: 1.49-4.26), and 1.76 (95% CI: 1.13-2.69), respectively.
Table 2.
Univariate and multivariate analyses for TTD.
| Variable | Reference group | TTD | |||||
|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
||||||
| HR | P value | 95% CI | HR | P value | 95% CI | ||
| Age ⩾70, y | <70 | 0.95 | .80 | 0.62-1.42 | |||
| PS >2 | 0/1 | 1.05 | .91 | 0.41-2.19 | |||
| PgR+ | PgR - | 0.85 | .45 | 0.58-1.29 | |||
| De novo metastatic disease: Yes | No | 1.03 | .88 | 0.69-1.50 | |||
| First line therapy: Yes | No | 0.44 | .0092 | 0.21-0.83 | 0.36 | .0029 | 0.16-0.72 |
| Number of metastatic sites is ⩾3 | <3 | 1.55 | .022 | 1.07-2.23 | |||
| Liver metastasis: Yes | No | 1.59 | .016 | 1.09-2.29 | 1.54 | .035 | 1.03-2.27 |
| Bone-only metastasis: Yes | No | 0.75 | .36 | 0.37-1.36 | |||
| LDH >300 | ⩽300 | 2.89 | <.001 | 1.68-4.67 | 2.58 | .0013 | 1.49-4.26 |
| NLR ⩾3.0 | <3.0 | 1.50 | .059 | 0.98-2.25 | 1.76 | .014 | 1.13-2.69 |
| History of everolimus: Yes* | No | 1.22 | .33 | 0.82-1.79 | |||
| History of CT: Yes* | No | 1.00 | .99 | 0.68-1.48 | |||
| RDI ⩾80% | <80% | 1.21 | .35 | 0.81-1.77 | |||
| RDI ⩾60% | <60% | 1.12 | .53 | 0.78-1.64 | |||
Abbreviations: CI, confidence interval; CT, chemotherapy; HR, hazard ratio; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PgR, progesterone receptor; PS, performance status; RDI, relative dose intensity; TTD, time-to-treatment discontinuation.
In patients who received a second- or later-line therapy.
Figure 2.
Kaplan-Meier curves of TTD for patients who received palbociclib with ET. (A) Patients who started with an initial palbociclib dose of 125 mg versus those who started with 100 mg or 75 mg. (B) Among patients who started with an initial palbociclib dose of 125 mg, the final dose was 125 mg versus 100 mg, 75 mg or 50 mg. (C) Patients who had RDI <80% versus those who had RDI ⩾80% and (D) patients who had RDI <60% versus those who had RDI ⩾60%.
ET indicates endocrine therapy; RDI, relative dose intensity; TTD, time-to-treatment discontinuation.
Safety
Hematologic AE data are shown in Table 3. Patients who experienced any grade of AE accounted for 97% of the total population, whereas 72% of the patients experienced grade 3-4 AEs. The most common hematologic AE was neutropenia, which occurred in 93% of patients. Leukopenia occurred in 92% of the patients, and the proportion of patients who experienced grade 3-4 AEs was 45%.
Table 3.
Hematologic adverse events.
| All grades (%) | Grades 3-4 (%) | |
|---|---|---|
| Leukopenia | 92.1 | 45.2 |
| Neutropenia | 92.7 | 71.2 |
| Anemia | 60.5 | 2.8 |
| Thrombocytopenia | 52.5 | 5.7 |
| Elevation of liver enzymes | 21.5 | 3.4 |
Discussion
In this study, we investigated the effectiveness, predictive factors, and safety of palbociclib. The median TTD in our cohort, for both the initial and latter lines of therapy, was considerably shorter than expected based on the results of the PALbociclib: ongoing trial in the management of breast cancer (PALOMA) studies.20,21 The effectiveness of palbociclib as a first-line therapy in our study was similar to that reported in a previous retrospective study by Varella et al22 (median PFS: 15.1 months). However, Wilkie et al23 reported a more prolonged median time-to-treatment failure (26.1 months) for patients treated with palbociclib plus aromatase inhibitor as a first-line therapy than that observed in our study. This difference may be attributed to the fact that liver metastasis results in poor outcomes, and the number of patients who had liver metastases in this study (50% of the first-line therapy patients) was greater than that of the previous study (10%). Even among patients who received palbociclib as a later-line therapy, the TTD observed in this study was shorter than that reported in a previous study.24 This may be due to the large number of patients receiving palbociclib as a third- or later-line therapy in this study.
Multivariate analysis showed that the following factors are significantly associated with more prolonged TTD: administration of palbociclib as the first-line therapy, no liver metastasis, low LDH level (⩽300 U/L), and low NLR (<3). Increase in serum LDH levels is known as an adverse prognostic factor in breast cancer and many malignancies.25 Elevated LDH is associated not only with tumor progression but also with alterations in the tumor microenvironment caused by the production of lactate, which induces local acidosis and lowers extracellular hydrogen potential (pH). Furthermore, various immunosuppressive effects of lactate have been reported.26 In contrast, low NLR indicates the strength of the immune response to the tumor and has attracted attention as an indicator of systemic immune response.15 Thus, both high LDH levels and NLR reflect an inhibition of the tumor-immune system and are associated with TTD. In preclinical studies, CDK4/6 inhibitors induced T-cell-related anti-tumor immunity, suggesting synergistic effects of combined treatment with CDK4/6 inhibitors and immunotherapy.27-30 These studies have also reported the vital effect of CDK4/6 inhibitor in the tumor microenvironment and provided the rationale for the new combination regimens composed of CDK4/6 inhibitors and immunotherapies. Further consideration will be needed to learn about the immune response in the systemic environment and the tumor microenvironment. Regarding other predictive factors, Pizzuti et al31 reported that PFS is positively affected by no everolimus/exemestane pretreatment for ABC. Conversely, pretreatment with everolimus was not associated with TTD in our univariate analysis. However, a direct comparison of the findings of these studies is difficult as our study has a smaller sample size.
The most frequent AE in this study, neutropenia, occurred in 93% of patients who had all grades of AE and in 71% of those who had grade 3-4 AEs. This result is consistent with the results of the analysis of neutropenia in Japanese patients conducted by Masuda et al.32 Although the occurrence of grade 3-4 neutropenia was high (71%), permanent discontinuation of palbociclib due to AE occurred in only 11% (20/177) of the cases in our cohort. This result is also comparable with that of a study of another Japanese cohort, which reported that 85% (22/26) of patients had grade 3-4 neutropenia and palbociclib was discontinued in 8% of cases (2/26).12 Regarding the association between dose reduction and effectiveness, no negative impact was recorded in this study and in the other Japanese report.32 Therefore, suitable dose adjustment could be allowed for clinical practice.
Our study has some limitations. First, this study had a smaller sample size, especially the number of patients who received palbociclib as a first-line therapy, and a shorter median follow-up period than the PALOMA studies. Second, there was a lack of data on potential confounding factors (including nonhematologic AEs and comorbidities) due to the retrospective nature of the study. Third, we used TTD to evaluate the effectiveness of palbociclib because we considered that the use of PFS could be biased due to the various intervals and evaluations used in imaging. Therefore, direct comparison with other clinical studies may be difficult. However, our study also has several strengths. We demonstrated the possibility of using serum LDH levels and NLR as predictive factors and reaffirmed that there is no association between dose reduction and duration of palbociclib treatment in a relatively large cohort of Japanese patients.
In conclusion, our real-world cohort, which included heavily pretreated patients, had a shorter treatment duration than expected based on the results of the pivotal phase 3 trials and other real-world studies. Our study also showed the possibility of using serum LDH levels and NLR as new predictive factors for HR+/HER2− ABC outcomes. Although the incidence of neutropenia in this study was high, suitable dose modification would not limit the benefits of palbociclib. To investigate the influence of a later line of therapy or visceral metastasis and use of serum LDH levels and NLR as reasonable predictive markers of palbociclib treatment outcomes, further prospective research is necessary.
Acknowledgments
We thank Prof. Kohei Akazawa for assistance with statistical analyses. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Y.K. received honoraria from Eisai, Novartis, Pfizer, Eli Lilly, Taiho, and Chugai. All other authors declare no conflict of interest.
Author Contributions: S.T. designed and supervised the study. S.T., Y.K., N.O., J.M., H.S., T.H., T.O., M.M., and M.S. contributed to data collection. N.O., Y.K., H.M., K.Y., and S.T. performed the analysis, prepared the figures, and wrote the manuscript. All authors discussed the results and contributed to the final manuscript.
ORCID iDs: Nina Odan  https://orcid.org/0000-0002-6952-3534
https://orcid.org/0000-0002-6952-3534
Masaru Miyashita  https://orcid.org/0000-0001-9463-4730
https://orcid.org/0000-0001-9463-4730
References
- 1. Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427-1438. [PubMed] [Google Scholar]
- 2. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25-35. [DOI] [PubMed] [Google Scholar]
- 4. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925-1936. [DOI] [PubMed] [Google Scholar]
- 5. Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209-219. [DOI] [PubMed] [Google Scholar]
- 6. Iwata H, Im SA, Masuda N, et al. PALOMA-3: phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer that progressed on prior endocrine therapy—safety and efficacy in Asian patients. J Glob Oncol. 2017;3:289-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukai H, Shimizu C, Masuda N, et al. Palbociclib in combination with letrozole in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int J Clin Oncol. 2019;24:274-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masuda N, Inoue K, Nakamura R, et al. Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int J Clin Oncol. 2019;24:262-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2019;37:1169-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masuda N, Nishimura R, Takahashi M, et al. Palbociclib in combination with letrozole as first-line treatment for advanced breast cancer: a Japanese phase II study. Cancer Sci. 2018;109:803-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwamoto N, Aruga T, Kuroi K, et al. Efficacy and toxicity of palbociclib in heavily treated patients with metastatic breast cancer. Ann Cancer Res. 2018;26:105-109. [Google Scholar]
- 13. Kikuchi M, Tanaka Y, Yokota M, et al. Analysis of the selection of CDK4/6 inhibitors based on experience using palbociclib. Biomed Rep. 2019;11:253-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu D, Wang D, Wu C, et al. Prognostic significance of serum lactate dehydrogenase in patients with breast cancer: a meta-analysis. Cancer Manag Res. 2019;11:3611-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faria SS, Fernandes PC, Jr, Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline update. J Clin Oncol. 2013;31:3997-4013. [DOI] [PubMed] [Google Scholar]
- 18. Marmorino F, Salvatore L, Barbara C, et al. Serum LDH predicts benefit from bevacizumab beyond progression in metastatic colorectal cancer. Br J Cancer. 2017;116:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwase T, Sangai T, Sakakibara M, et al. An increased neutrophil-to-lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol Clin Oncol. 2017;6:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926-1936. [DOI] [PubMed] [Google Scholar]
- 21. Rugo HS, Finn RS, Dieras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varella L, Eziokwu AS, Jia X, et al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res Treat. 2019;176:429-434. [DOI] [PubMed] [Google Scholar]
- 23. Wilkie J, Schickli MA, Berger MJ, et al. Progression-free survival for real-world use of palbociclib in hormone receptor-positive metastatic breast cancer. Clin Breast Cancer. 2020;20:33-40. [DOI] [PubMed] [Google Scholar]
- 24. Bui TBV, Burgers DM, Agterof MJ, van de Garde EM. Real-world effectiveness of palbociclib versus clinical trial results in patients with advanced/metastatic breast cancer that progressed on previous endocrine therapy. Breast Cancer (Auckl). 2019;13:1178223418823238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961-970. [DOI] [PubMed] [Google Scholar]
- 26. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19:353-363. [DOI] [PubMed] [Google Scholar]
- 27. Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8:216-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaer DA, Beckmann RP, Dempsey JA, et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22:2978-2994. [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Bu X, Wang H, et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature. 2018;553:91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pizzuti L, Giordano A, Michelotti A, et al. Palbociclib plus endocrine therapy in HER2 negative, hormonal receptor-positive, advanced breast cancer: a real-world experience. J Cell Physiol. 2019;234:7708-7717. [DOI] [PubMed] [Google Scholar]
- 32. Masuda N, Mukai H, Inoue K, et al. Neutropenia management with palbociclib in Japanese patients with advanced breast cancer. Breast Cancer. 2019;26:637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]