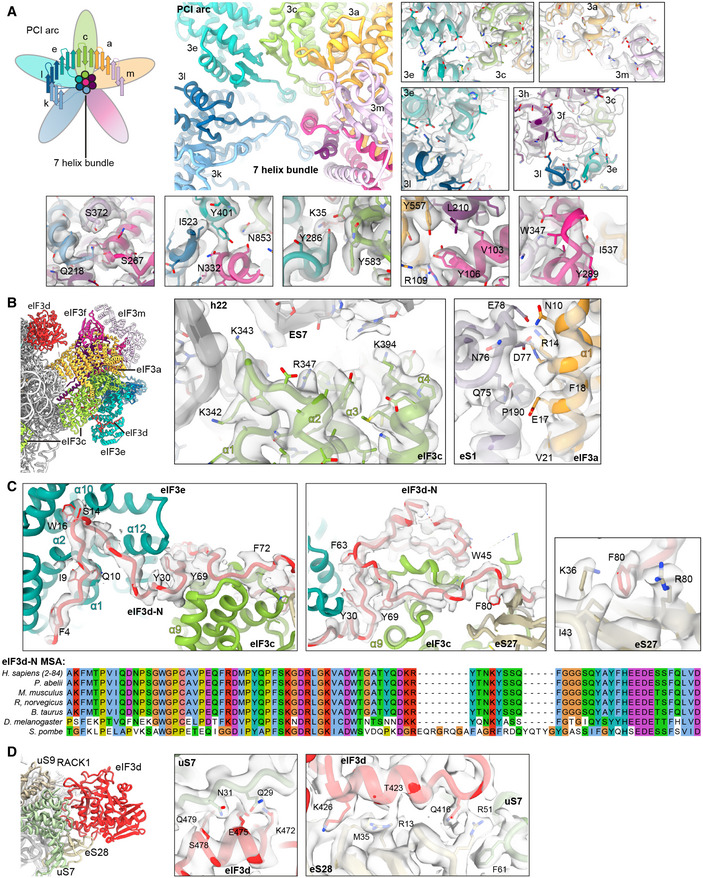
Left, Middle: Interactions of the eIF3d N‐terminal tail with the PCI‐MPN core: Interactions of the ultimate eIF3d N‐terminus (Phe4‐Pro18) with eIF3e are established
via Phe4 (to Tyr32 in loop between PCI helices α2 and α3), Gln10 (to His12 in α1), Ile9 and Asp11 (to Arg16 in α2), Ser14 (to Asn164 α9 and Phe132 in α7), Trp16 (to Gly171 in α10), and Gly17 (to Trp170 in α10). eIF3d residues 25‐36 are bridging eIF3e and eIF3c. Residues involved are Tyr30 (to Leu208 in eIF3e PCI helix α12), Phe33 (to the peptide backbone of Leu590 in eIF3c α11), and Lys35 (to Gln283 in eIF3e α16). eIF3c‐specific interactions are established by Leu39 (to Gln595), Trp45 (Pro603, Ile607, and Glu666), and Thr46 (to Arg641) (see Table
S3). For clarity, only density for eIF3d is shown (gray transparent surface). Right: Detailed view of the interaction of F80 (eIF3d) with K36 and R80 (eS27). Density is shown for both proteins (gray transparent surface). Below: MSA of the conserved N‐terminal region of eIF3d is shown. Coloring according to default Clustal X color scheme (blue: hydrophobic, magenta: negative charge, red: positive charge, green: polar, orange: glycine, yellow: proline, pink: cysteine, cyan: aromatic).