Abstract
Background: Rio de Janeiro and Niterói municipalities in southeastern Brazil experience large dengue epidemics every 2 to 5 years, with >100,000 cases notified in epidemic years. Costs of vector control and direct and indirect costs due to the Aedes-borne diseases dengue, chikungunya and Zika were estimated to total $650 million USD in 2016, but traditional vector control strategies have not been effective in preventing arboviral disease outbreaks. The Wolbachia method is a novel and self-sustaining approach for the biological control of arboviral diseases, in which the transmission potential of Ae. aegypti mosquitoes is reduced by stably transfecting them with the Wolbachia bacterium. This paper describes a study protocol for evaluating the effect of large-scale non-randomised releases of Wolbachia mosquitoes on the incidence of dengue, Zika and chikungunya in the municipalities of Niterói and Rio de Janeiro. This follows a lead-in period since 2014 involving intensive community engagement, regulatory and public approval, entomological surveys, and small-scale pilot releases.
Method: The planned releases during 2017-2019 cover a combined area of 121 km2 with a resident population of 1.1 million, across the two cities. Untreated areas with comparable historical dengue profiles and sociodemographic characteristics have been identified a priori as comparative control areas in each municipality. The proposed pragmatic epidemiological approach combines a controlled interrupted time series analysis of routinely notified suspected and laboratory-confirmed arboviral cases, together with monitoring of arbovirus activity utilising outbreak signals routinely used in public health disease surveillance.
Discussion: If the current project is successful, this model for control of arboviral disease through Wolbachia releases can be expanded nationally and regionally.
Keywords: Wolbachia, dengue, chikungunya, Zika, vector-borne disease, disease surveillance, controlled interrupted time series, Brazil
Abbreviations
BG trap: BG-Sentinel trap; IBGE: Instituto Brasileiro de Geografia e Estatística (Brazilian Institute of Geography and Statistics); ITS: interrupted time series; MoH: Ministry of Health; PAHO: Pan American Health Organisation; PCR: polymerase chain reaction; qPCR: quantitative polymerase chain reaction; RCT: randomised controlled trial; SINAN: Sistema De Informação De Agravos De Notificação (Brazilian National Notifiable Diseases Information System); WHO: World Health Organisation
Background
The global incidence of dengue has increased dramatically in recent decades. Although cases are underreported, it is estimated that 390 million dengue virus infections occur every year, and of these 96 million have clinical manifestations of dengue or severe dengue. Globally, 3.9 billion people in 128 countries are at risk of infection 1. The primary vector of dengue is the Aedes aegypti mosquito, which is also capable of transmitting other arboviruses including chikungunya, Zika, yellow fever and Mayaro 2.
The first reported dengue outbreak in Brazil was in 1845, with subsequent outbreaks in 1880–1912 and 1916–1923 2. As a result of a coordinated effort from the Pan American Health Organization (PAHO) and the World Health Organization (WHO) to eradicate Ae. aegypti, Brazil was considered free of the mosquito in 1955, but the vector was reintroduced into the country two decades later 3. In 1986, dengue virus serotype 1 (DENV1) was introduced to Rio de Janeiro and an estimated 1 million people were infected 3, 4. Since then, dengue has become a major public health problem. From 1986 to 1993, outbreaks occurred approximately every 2–5 years, and from 1993 dengue became endemic with seasonal peaks in cases during the rainy season (December to May), but with ongoing transmission throughout the year 2, 3. Between 2000 and 2007, more than 3 million dengue cases were reported (caused by DENV serotypes 1, 2 and 3) and in 2010, DENV4 re-emerged after 28 years of absence 2.
The recent introduction of Zika and chikungunya in dengue hyperendemic areas of Brazil has aggravated the situation. The overlapping clinical features, absence of serological assays for the Zika virus that can reliably distinguish between acute disease and past exposure, and the association of pregnancy-associated Zika virus infection with microcephaly and other neurologic complications represents a great challenge for public health that will require new strategies and innovations 5, 6.
Between 2016 and 2017, 762 deaths were attributed to severe dengue in Brazil 7. Additionally, in 2017, 127 deaths were confirmed to be caused by chikungunya. Yellow fever has spread from the North of Brazil to the Southeast over the last years, affecting humans and non-human primates. From July 2017 to April 2018, 1,266 cases of yellow fever including 415 deaths were confirmed in Brazil, with 223 cases and 73 deaths occurring in Rio de Janeiro State 8. No autochthonous cases were reported in Rio de Janeiro city or Niterói, although some residents from those cities acquired yellow fever while traveling to other places in Brazil. A mass vaccination campaign against yellow fever began in 2016.
The costs of vector control, direct medical costs, and indirect costs related to dengue, Zika and chikungunya in Brazil were estimated to be 2.3 billion Brazilian reais ($650 million USD) in 2016 9. In the absence of an effective vaccine for these arboviruses, disease prevention depends on vector control. Vector control guidelines in Brazil 10 are focused on elimination or larvicide treatment of mosquito breeding sites and the control of adult mosquito populations with insecticides sprayed as ultra-low volume. The limited potential of these traditional vector control strategies to achieve large-scale and sustained reductions in dengue incidence is evidenced by the continuing public health burden of dengue throughout endemic areas where these measures are routinely employed, and the lack of robust efficacy data from well-designed trials to inform their optimal implementation 11.
The Wolbachia method ( www.worldmosquito.org) is a novel, natural, and self-sustaining approach to reduce arboviral diseases transmitted by Ae. aegypti mosquitoes. The symbiotic Wolbachia bacterium is found naturally in over 60% of insect species, but not in Ae. aegypti, and is passed from one generation to the next through the insect’s eggs . Stable transinfection of Wolbachia into a local Ae. aegypti colony in the laboratory produces a lineage of Wolbachia-carrying mosquitoes which, upon release over several weeks, can achieve dissemination of Wolbachia into the local Ae. aegypti population through the processes of maternal inheritance and cytoplasmic incompatibility that give Wolbachia-carrying mosquitoes a reproductive advantage. The DENV-transmitting potential of Wolbachia infected mosquitoes is reduced by 66–75% 12, 13, a phenotype which has been shown to persist in field mosquito populations up to five years after the end of releases. Mathematical modelling of this reduced transmissibility predicts a substantial and sustained reduction in dengue incidence in human populations where Wolbachia is established 13. Laboratory data indicate a similar reduction in the competence of Wolbachia-carrying Ae. aegypti for transmitting other viruses including Zika, chikungunya, yellow fever and Mayaro 14– 17.
With releases now conducted in eight countries over the past eight years, the World Mosquito Program has demonstrated that Wolbachia can be successfully established and maintained in both small-scale and large-scale urban settings in multiple ecological environments 18– 22.
A core objective of these releases has been to ensure strong community acceptance and government support for the approach, achieved through embedding community and stakeholder engagement within the project activities in each site. Observational evidence of the impact of Wolbachia releases on arboviral disease in pilot sites has been encouraging, with no evidence of local dengue transmission where Wolbachia has established at high levels. Following city-wide deployment in Townsville, Australia, there has been no confirmed local dengue transmission in Wolbachia-treated areas for four seasons since completion of releases, despite local transmission every year for the prior 13 years and ongoing importation of DENV infection in travelers 21. A cluster randomized controlled trial (RCT) to generate a robust and quantitative estimate of the impact of Wolbachia on dengue incidence commenced in Yogyakarta, Indonesia in 2017, with reporting of results expected in 2021 23.
In Brazil, planned scale up of Wolbachia deployments from demonstration projects in Rio de Janeiro and Niterói municipalities to large-scale releases was accelerated by the declaration of Zika as a public health emergency by the WHO in early 2016, and the recommendation by WHO’s Vector Control Advisory Group in March 2016 that the Wolbachia method be evaluated in rigorously monitored pilot deployments under operational conditions, to build evidence of epidemiological effectiveness against Aedes-borne viruses 24. Given the imperative from stakeholders and funders to scale up deployment within a relative short time frame, and to retain sufficient flexibility to optimize methods for large-scale deployment in the varied micro-environments within Niterói and Rio de Janeiro, an RCT or other carefully controlled deployment was not considered feasible. Instead releases under operational conditions, and with pragmatic evaluation of disease impact using data routinely collected for public health purposes, was favoured.
Here we describe a protocol for evaluating the effect of large-scale non-randomized Wolbachia releases on the incidence of dengue, Zika and chikungunya in the municipalities of Niterói and Rio de Janeiro. The proposed strategy employs a controlled interrupted time series analysis of routinely notified suspected and laboratory-confirmed arboviral cases, together with monitoring of arbovirus activity with outbreak signals routinely used in public health disease surveillance. This methodology allows measurement of the impact of the intervention at the population level over time, accounting for the seasonal trends and inter-annual fluctuations often observed in dengue and other arboviral disease incidence.
Methods
Study design
The aim of this epidemiological study is to test the hypothesis that the establishment of Wolbachia in local Ae. aegypti populations in Rio de Janeiro and Niterói leads to a reduction in arboviral disease burden.
The impact of Wolbachia deployment on arboviral disease incidence will be evaluated using routine notifiable disease surveillance data to describe associations between temporal and spatial trends in arbovirus disease and the deployment of Wolbachia across Niterói and Rio de Janeiro, with two objectives:
-
1.
Estimate the reduction in dengue incidence in the aggregate treated areas of Niterói and Rio de Janeiro compared to an untreated control area, and in each treated zone compared to the untreated control area, each year for five years after Wolbachia establishment.
-
2.
Quantify the occurrence of dengue outbreak signals in Wolbachia treated areas compared to untreated areas in Niterói and Rio de Janeiro, for five years after Wolbachia establishment, using outbreak indicators employed routinely for public health monitoring of dengue activity: i) control diagrams comparing the weekly dengue incidence (five-week moving average) against the five-year historical average in that same area; and ii) outbreak incidence threshold of 300/100,000 population in any month.
Study setting and population
Rio de Janeiro and Niterói cities are located in the State of Rio de Janeiro, Brazil. Niterói has an area of 134 km 2 and a population of 487,562 in 2010. Rio de Janeiro is the second largest city in Brazil with 6,320,446 inhabitants in 2010 and an area of 1,200 km 2. The two cities sit on opposite sides of the Guanabara Bay and are linked by a long bridge, which transports a large commuter population between Niterói and Rio de Janeiro.
The cities are divided into health districts, for the purpose of planning and delivering care - ten in Rio de Janeiro and seven in Niterói. In Rio de Janeiro, Wolbachia deployments will be conducted in one of the administrative areas of the city ( Figure 1; produced in ArcMap version 10.5, ESRI, CA), in an area of approximately 90 km 2 and with 886,551 inhabitants, 39% of whom live in slums. The total release area in Niterói is approximately 31 km 2 covering a population of 268,536 ( Table 1). For the purposes of Wolbachia deployment, the Rio de Janeiro and Niterói intervention areas are divided into 4 and 3 release zones, respectively, which are aligned with neighbourhood administrative boundaries.
Figure 1.
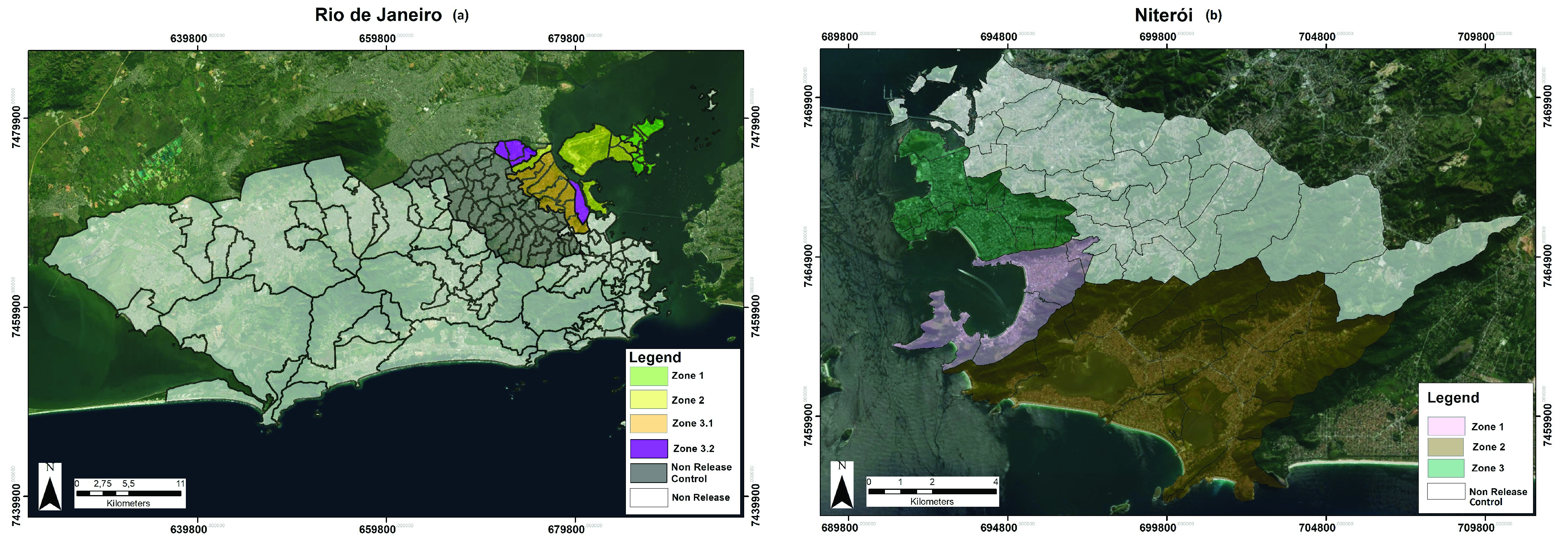
Map of ( a) Rio de Janeiro and ( b) Niterói Wolbachia-treated and untreated areas (produced in ArcMap version 10.5, ESRI, CA).
Table 1. Sociodemographic characteristics of Wolbachia treated and untreated areas, Niterói and Rio de Janeiro (source: 2010 Brazil population census).
| Neighbourhoods | Population | Area (km 2) | Population density | |
|---|---|---|---|---|
| Niterói | ||||
| Zone 1 | 4 | 23,747 | 9.2 | 2581 |
| Zone 2 | 11 | 68,695 | 50.6 | 1357 |
| Zone 3 | 13 | 178,891 | 12.6 | 14,197 |
| Non-release control area | 24 | 216,229 | 62.1 | 3481 |
| Total | 52 | 487,562 | 134.5 | 3624 |
| Rio de Janeiro | ||||
| Zone 1 | 10 | 107,130 | 11.8 | 9078 |
| Zone 2 | 6 | 150,646 | 33.5 | 4496 |
| Zone 3.1 | 8 | 408,036 | 32.6 | 12,516 |
| Zone 3.2 | 4 | 220,739 | 12.0 | 18,394 |
| Non-release control area | 52 | 1,512,608 | 117.3 | 12,895 |
| Non-release area | 80 | 3,921,287 | 996.5 | 3935 |
| Total | 160 | 6,320,446 | 1203.7 | 5250 |
In Rio de Janeiro, two administrative areas adjacent to the release area have been designated a priori as a comparative control zone ( Figure 1), based on comparable sociodemographic characteristics and synchronous historical dengue time series ( Figure 2 and Table 1). In Niterói, the remaining untreated area of the city has been designated as the comparative control zone ( Figure 1).
Figure 2.
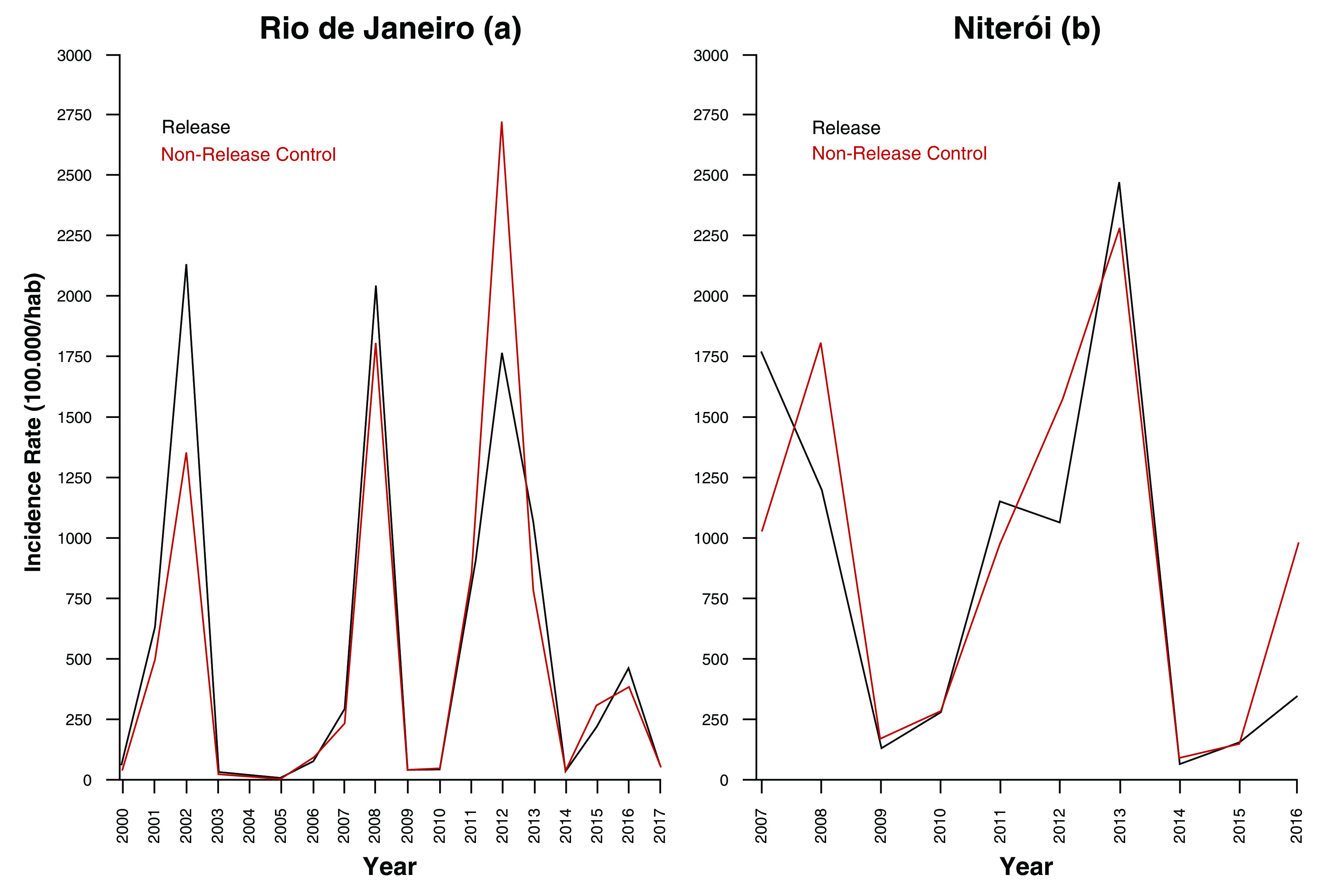
Dengue incidence in Wolbachia-treated vs untreated areas of ( a) Rio de Janeiro (2000–2017) and ( b) Niterói (2007–2016) (source: Brazilian National Disease Surveillance System (SINAN)).
Wolbachia release and monitoring
Staged Wolbachia-mosquito deployments will be implemented in Niterói and Rio de Janeiro, in order to achieve Wolbachia establishment across the two municipalities. Pilot releases commenced in late 2015, and city-wide deployments are ongoing through to the end of 2019. Wolbachia-containing adult mosquitoes will be released at one location per 50 x 50 meter grid square for a minimum of 16 consecutive weeks in each release zone. In areas where Wolbachia frequency remains low after 16 weeks of releases, or where particularly high wild type Ae. aegypti populations are observed, Wolbachia-containing mosquito eggs will also be released to complement adult releases with the aim of accelerating Wolbachia establishment. Monitoring of Wolbachia frequency will be done using BG-Sentinel mosquito traps (BioGents, Germany), distributed throughout the release area at a density of 16 traps per km 2. Traps will be serviced weekly and all collected mosquitoes identified morphologically by microscopy. From eight weeks after the start of releases, a maximum of 10 Ae. aegypti (male and female) per trap will be tested individually for Wolbachia using quantitative polymerase chain reaction (qPCR). Wolbachia screening will be performed biweekly during releases and until establishment, then every 1–3 months thereafter. All surplus Ae. aegypti will be biobanked. Mosquito collection and screening results will be stored in a custom designed web-based data repository. The Wolbachia prevalence in screened Ae. aegypti will be reported aggregated to each release zone, calculated as the total number of Ae. aegypti mosquitoes that tested positive for Wolbachia aggregated across all BG traps in the zone, divided by the total number of Ae. aegypti that were screened in that zone.
Epidemiological data sources
The two proposed strategies for the evaluation of the impact of large-scale Wolbachia deployments on arboviral disease incidence in Niterói and Rio de Janeiro municipalities make use of existing data on dengue, chikungunya and Zika case notifications to the Brazilian national disease surveillance system (SINAN). Dengue surveillance has been in place since its re-emergence in 1986 and data is available from the SINAN system since 2000. Zika and chikungunya became notifiable diseases in 2015.
Suspected cases of dengue and other arboviral diseases are required to be reported to the city health department 25, according to a case definition of fever plus two other symptoms including malaise, headache, myalgia, nausea, vomiting, cutaneous rash, and arthralgia. Dengue case notifications include an indication of disease severity (dengue, dengue with alarm signals, severe dengue, fatal dengue). A variable proportion of notified suspected cases are tested by IgM serology or PCR, following the Brazilian guidelines 26 and the timing between onset of symptoms and blood collection. The number of cases in a given period of time may limit the availability of tests, and PCR testing is routinely performed only for severe and fatal cases, pregnant women and young children 26. In 2016, 4.8% of notified dengue cases in Rio de Janeiro and 11.5% in Niterói were supported by a positive IgM serology result, and only 0.2% of notified cases in Rio and 0.03% in Niterói had a positive PCR result. The ability to confirm dengue cases by serology is impaired since the Zika outbreak due to serological cross-reactivity between the dengue and Zika viruses. As PCR testing is performed only in certain patient populations, the proportion positive is unlikely to be generalisable to all notified cases. Therefore, for the purpose of our analyses we will use all notified dengue cases (suspected and laboratory-confirmed) as the primary endpoint.
In the absence of a reliable serological test that does not cross-react with dengue, Zika lab diagnosis is done solely on the basis of molecular detection (real-time PCR) up to the first 5 days in serum and 15 days in urine. This has severely limited the ability to confirm Zika virus infection among notified cases (3.5% in 2016, 19.3% in 2017).
Chikungunya diagnoses can be confirmed either through PCR or serology, as it does not cross-react with Zika or dengue. The proportion of notified cases with supportive laboratory findings is higher than for dengue: 31.9% and 18.2% in Rio and Niterói, respectively, in 2016.
Anonymized disaggregate (line-listed) data on notified suspected and laboratory-confirmed dengue, chikungunya and Zika cases will be extracted from the SINAN system for the historical pre-intervention period (2000–2016 for dengue, and 2015–2016 for chikungunya and Zika) and the prospective post-intervention period (2017–2023). The dataset will include age, sex, neighbourhood of primary residence, date of illness onset, date of notification, reporting health clinic, disease severity, hospitalisation, death, and where available, geocoordinates of primary residence, type of diagnostic test performed, diagnostic test result, and final diagnostic classification. Population data from the Brazilian census (IBGE) and population by neighbourhood of residence obtained from the municipalities of Rio and Niterói will be used to estimate the population in each release zone. The incidence rate (number of new dengue, Zika or chikungunya cases divided by the population at risk) will be expressed per 100,000 inhabitants.
Controlled interrupted time series analysis
Interrupted time series (ITS) analysis is a valuable study design for evaluating the effectiveness of a population-level health intervention that is implemented at a clearly defined point in time 27. It uses a set of historical observations of an outcome of interest (in this context monthly arboviral case notifications) to establish an underlying trend, which is assumed to be ‘interrupted’ by the introduction of an intervention (in this case Wolbachia releases). Descriptive analyses of seasonal and interannual trends in arboviral disease notifications, by release zone and municipality, will first be made. Comparison of the trend in monthly case notifications in the post-intervention period with the hypothetical scenario of no intervention (the ‘counterfactual’, inferred from the historical time series and untreated control area), provides an estimate of the intervention effect. Segmented regression using an appropriately defined impact model (e.g. negative binomial regression for autocorrelated count data with population offset, assuming a step change post-intervention) will be used to estimate the effect of Wolbachia releases on the arboviral disease endpoints in each release zone, and for the aggregate release areas in each municipality. In Rio and Niterói, the availability of comparative control areas – well-matched to the release area in socioeconomic characteristics and historical dengue incidence ( Figure 2) – permits a robust, controlled analysis in which the confounding effects of seasonality and inter-annual variability can be adjusted for. Zone-level ITS analyses will be performed 12 months after completion of releases in each zone, and each 12 months thereafter, with release zones considered ‘treated’ for the purpose of this analysis based on completion of releases, regardless of the long-term Wolbachia monitoring results. Aggregate release-area level analyses will be performed for each municipality 12 months after completion of releases in the last zone and each 12 months thereafter.
Power was estimated for the ITS analysis using 1000 simulated datasets drawn from a negative binomial distribution fitted to a ten-year time series (2007–2016) prior to Wolbachia deployment, of monthly dengue case notifications from release and control zones in Niterói and Rio de Janeiro. The simulated time series of dengue case numbers in the control zones as well as the pre- Wolbachia release dengue case numbers in the treated zones were drawn directly from this model-generated distribution. Post- Wolbachia release dengue case numbers in the treated zones were drawn from the same model-generated distribution, modified by an additional parameter for an intervention effect of Relative Risks = 0.6, 0.5, 0.4, 0.3. For each of these four ‘true’ effect sizes and a null effect (RR = 1), applied to each of the 1000 simulated time series, the ‘observed’ effect size was calculated from a negative binomial regression model of monthly case counts in the treated and untreated zones, as described above. Post-intervention time periods of 1, 2 or 3 years were simulated, with the pre-intervention period fixed at 7 years. The estimated power to detect a given effect size was determined as the proportion of the 1000 simulated scenarios in which a significant intervention effect (p<0.05) was observed. These simulations indicate 80% power to detect a reduction in dengue incidence of 50% or greater after three years of post-intervention observations, and a reduction of 60% or greater after two years.
The primary endpoint for the ITS analysis will be dengue cases notified to the disease surveillance system. The secondary endpoints will be: a) the count of severe dengue cases reported to the surveillance system, b) the count of fatal dengue cases reported to the surveillance system, and c) Zika and chikungunya cases notified to the disease surveillance system.
Although the historical time series for Zika and chikungunya incidence is short, we will nonetheless describe the incidence during and after Wolbachia deployments relative to the a priori defined non-release control areas for both Niterói and Rio de Janeiro.
Dengue outbreak signals
As a complementary approach for evaluating the public health impact of large-scale Wolbachia releases, we will also use the following dengue outbreak alert tools routinely used in public health practice. We hypothesise that these dengue outbreak signals will not be triggered in areas where Wolbachia has been established.
1. Control diagram (endemic channel)
The definition of a dengue outbreak or epidemic has changed over time in Brazil. In Rio de Janeiro and Niterói, a control diagram is currently used to monitor dengue incidence. Briefly, the control diagram is constructed from a five-week centered moving average of weekly notified dengue incidence for the past five years excluding epidemic years. An early signal of a dengue outbreak/epidemic is triggered when the weekly incidence of dengue crosses the upper limit of the control diagram 26, with the upper limit defined as [mean+(standard deviation*1.96)]. Incidence that remains above the upper limit of the control diagram for two or more consecutive weeks constitutes a dengue outbreak. For the purpose of monitoring the impact of Wolbachia releases on dengue, we will construct annual control diagrams with weekly dengue incidence, by city and for each release and non-release zone, to monitor the occurrence of dengue outbreaks. The number of dengue outbreak signals triggered per year will be reported.
2. Classical incidence threshold
Another outbreak definition that has been used by the Ministry of Health (MoH) in previous years 20, 22, 23 is a dengue incidence threshold of ≥300 cases/100,000 population in a given month. Although not included in current MoH guidelines, this provides an alternative endpoint for evaluating dengue activity at a population level in the post-intervention period, compared with pre-intervention, and we will report the number of months in a given year where dengue incidence crosses this threshold.
Current study status
This study is ongoing. Wolbachia releases are expected to be completed by the end of 2019, and the collation and analysis of disease surveillance data will continue until 2023.
Dissemination of study results
Based on the results of the power estimation above, the study outcome will be evaluated and reported two years after the completion of releases. The findings will be submitted for peer review and publication in an appropriate open access journal, together with aggregate supporting data.
Discussion
The municipalities of Rio de Janeiro and Niterói in southeastern Brazil have been affected by dengue for more than 30 years, with epidemics occurring every 2 to 5 years. In recent years, outbreaks of the other Aedes-borne diseases chikungunya and Zika have presented further public health challenges, and since July 2017 at least 221 human cases of yellow fever have been confirmed in Rio de Janeiro State, including in urban areas 8. Vector control strategies, based on elimination of mosquito breeding sites and use of insecticides to reduce adult populations, have not been effective in preventing dengue outbreaks 28. The Wolbachia method is a novel and self-sustaining approach for the biological control of arboviral diseases. The signature feature of Wolbachia is to reduce the arbovirus-transmission potential of Wolbachia-infected mosquitoes 12, 14, 16, 17. The World Mosquito Program 29 is deploying Wolbachia-infected Ae. aegypti mosquitoes in Brazil with the purpose of achieving a large-scale and sustained reduction in arboviral disease burden in two cities where these diseases are public health priorities.
In March 2016, the WHO convened a Vector Control Advisory Group to review new and existing vector control tools for use in the response to the Zika virus outbreak. Based on the available evidence that Wolbachia reduces the Zika, dengue and chikungunya transmission potential of Ae. aegypti mosquitoes and field data showing long-term establishment of Wolbachia in mosquito populations in a range of environmental settings, the WHO recommended carefully monitored pilot implementation of the Wolbachia method in affected countries 24.
While RCTs are still considered the gold standard, they are not always feasible or agreeable to the community and government. The controlled ITS analysis is a quasi-experimental design that is commonly used to evaluate population-level public health intervention 27, 30, 31 and is a pragmatic alternative design where an RCT is considered infeasible, particularly in the presence of a well-matched untreated control area 32, 33. Given the public health emergency posed by the Zika epidemic at the time of this study’s inception and the need to scale up Wolbachia deployment in Rio de Janeiro and Niterói within a relative short time frame, an RCT or other carefully controlled deployment was not considered feasible. The controlled ITS is appropriate for the pragmatic evaluation of large-scale Wolbachia deployments given the long and reliable time series of dengue mandatory reporting data from both Rio de Janeiro and Niterói that allows for a longitudinal assessment of dengue trends before and after the Wolbachia intervention. Assessment of an impact of the intervention on chikungunya and Zika may be more difficult given their shorter time series.
Notifiable disease surveillance data can be limited by a lack of specificity in case definitions and inconsistent reporting practices, which may influence our ability to detect a true intervention effect on arboviral disease incidence. A subset of notified dengue cases are supported by laboratory diagnostic results, but these have several limitations: i) laboratory testing occurs infrequently (<15% of notified cases), particularly during outbreaks, ii) the cross-reactivity of IgM serology between dengue and Zika limits the utility of serological data since 2015, and iii) the restricted use of PCR in only certain patient populations limits the generalisability of PCR-positivity rates to all notified cases. We therefore base our analyses on all notified cases (suspected and confirmed). Benefits of using these routinely collected data include the availability of a long time series, reduced costs for data collection and timely acquisition of data.
Human mobility also presents a challenge to this study as individuals are likely to spend time in both Wolbachia-treated and untreated areas, making it difficult to determine the place of illness acquisition among notified cases of arboviral disease. Travel between areas means that the true Wolbachia exposure status of individuals resident in Wolbachia-treated and untreated areas becomes more similar, thereby diluting the observed intervention effect towards the null.
The introduction of Zika and chikungunya viruses brought new challenges to health surveillance and a greater willingness for better vector control in affected regions. With the re-emergence of yellow fever in Brazil, there is even more potential public health benefit if the Wolbachia intervention successfully reduces the vector competence of Ae. aegypti mosquitoes in the field and reduces arboviral disease incidence. No specific treatment for dengue, chikungunya or Zika currently exists. Although a vaccine against dengue (Sanofi Dengvaxia ®) was licensed in 2015, it was recently found to enhance the severity of subsequent dengue infection in individuals who were seronegative at the time of vaccination. As a result, a serology test prior to the administration of the vaccine is required to confirm previous dengue infection, increasing costs and decreasing feasibility in high-burden areas 34.
Releases of Wolbachia are completed or underway in eight countries, with no evidence of local transmission of dengue, Zika or chikungunya in places where Wolbachia is established at high levels 21. The implementation of the Wolbachia intervention is complex and has not been done on a large scale in very densely populated urban areas in the Americas before. The implementation of the project was preceded by careful work to engage the community and gain public acceptance for the intervention, even in the most difficult contexts of poverty and urban violence present in both Rio de Janeiro and Niterói. Engagement, entomological monitoring, and public health impact assessment activities were developed in close partnership with local governments. If the current project is successful, this model can be expanded to the rest of the country and the Americas.
Ethics approval and consent to participate
The study was approved by the Brazilian Institutional Review Board (Conep) (CAAE: 59175616.2.0000.0008). The study uses pre-existing non-identifiable disease surveillance data, which does not require individuals’ consent.
Data availability
No data is associated with this article.
Acknowledgements
The authors acknowledge the contributions of Peter Ryan, Simon Kutcher, Jacqui Montgomery, and Frederico Muzzi (World Mosquito Program) to the planning and implementation of Wolbachia deployments in Rio de Janeiro and Niterói.
Funding Statement
This study is funded by the Bill and Melinda Gates Foundation (OPP1099252); the Brazilian Ministry of Health (DECIT; TED 09/2016); and the US Centers for Disease Control (CDC).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved
References
- 1. Bhatt S, Gething PW, Brady OJ, et al. : The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brathwaite Dick O, San Martín JL, Montoya RH, et al. : The history of dengue outbreaks in the Americas. Am J Trop Med Hyg. 2012;87(4):584–93. 10.4269/ajtmh.2012.11-0770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fares RC, Souza KP, Añez G, et al. : Epidemiological Scenario of Dengue in Brazil. Biomed Res Int. 2015;2015:321873, Accessed 4 Jul 2018. 10.1155/2015/321873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siqueira JB, Jr, Martelli CM, Coelho GE, et al. : Dengue and dengue hemorrhagic fever, Brazil, 1981-2002. Emerg Infect Dis. 2005;11(1):48–53. 10.3201/eid1101.031091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brasil P, Calvet GA, Siqueira AM, et al. : Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLOS Negl Trop Dis. 2016;10(4):e0004636. 10.1371/journal.pntd.0004636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mlakar J, Korva M, Tul N, et al. : Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374(10):951–8. 10.1056/NEJMoa1600651 [DOI] [PubMed] [Google Scholar]
- 7. Boletins epidemiológicos. Secretaria de Vigilância em Saúde, Ministéio da Saúde.2018; Accessed 5 Jul 2018. Reference Source [Google Scholar]
- 8. Monitoramento do Período Sazonal da Febre Amarela Brasil - 2017/2018. Ministry of Health. Acessed 28 Jan 2019. Reference Source [Google Scholar]
- 9. Teich V, Arinelli R, Fahhan L: Aedes aegypti e sociedade: o impacto econômico das arboviroses no Brasil. JBES: Brazilian Journal of Health Economics. 2017;9(3):267–76. 10.21115/JBES.v9.n3.p267-76 [DOI] [Google Scholar]
- 10. Controle de vetores.2018; Accessed 26 Nov 2018. Reference Source [Google Scholar]
- 11. Wilson AL, Boelaert M, Kleinschmidt I, et al. : Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015;31(8):380–90. 10.1016/j.pt.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 12. Ye YH, Carrasco AM, Frentiu FD, et al. : Wolbachia Reduces the Transmission Potential of Dengue-Infected Aedes aegypti. PLoS Negl Trop Dis. 2015;9(6):e0003894. 10.1371/journal.pntd.0003894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferguson NM, Kien DT, Clapham H, et al. : Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7(279):279ra37. 10.1126/scitranslmed.3010370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dutra HL, Rocha MN, Dias FB, et al. : Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe. 2016;19(6):771–4. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aliota MT, Peinado SA, Velez ID, et al. : The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep. 2016;6:28792. 10.1038/srep28792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Hurk AF, Hall-Mendelin S, Pyke AT, et al. : Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6(11):e1892. 10.1371/journal.pntd.0001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pereira TN, Rocha MN, Sucupira PHF, et al. : Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci Rep. 2018;8(1):6889. 10.1038/s41598-018-25236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Neill SL, Ryan PA, Turley AP, et al. : Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses [version 2; peer review: 2 approved]. Gates Open Res. 2018;2:36. 10.12688/gatesopenres.12844.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffmann AA, Montgomery BL, Popovici J, et al. : Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–7. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 20. Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, et al. : Stability of the wMel Wolbachia Infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis. 2014;8(9):e3115. 10.1371/journal.pntd.0003115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt TL, Barton NH, Rašić G, et al. : Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017;15(5):e2001894. 10.1371/journal.pbio.2001894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia GA, Sylvestre G, Aguiar R, et al. : Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl Trop Dis. 2019;13(1):e0007023. 10.1371/journal.pntd.0007023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anders KL, Indriani C, Ahmad RA, et al. : The AWED trial (Applying Wolbachia to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster randomised controlled trial. Trials. 2018;19(1):302. 10.1186/s13063-018-2670-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. WHO: Mosquito (vector) control emergency response and preparedness for Zika virus. Accessed 24 Oct 2018. Reference Source [Google Scholar]
- 25. Ministério da Saúde.2016; Accessed 25 Oct 2018. Reference Source [Google Scholar]
- 26. Guia de vigilância epidemiológica. Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. - 7. ed. - Brasília: Ministério da Saúde.2009; Accessed 25 Oct 2018. Reference Source [Google Scholar]
- 27. Bernal JL, Cummins S, Gasparrini A: Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–55. 10.1093/ije/dyw098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gubler DJ: Dengue, Urbanization and Globalization: The Unholy Trinity of the 21 st Century. Trop Med Health. 2011;39(4 Suppl):3–11. 10.2149/tmh.2011-S05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Mosquito Program. Accessed 25 Oct 2018. Reference Source [Google Scholar]
- 30. Gasparrini A, Gorini G, Barchielli A: On the relationship between smoking bans and incidence of acute myocardial infarction. Eur J Epidemiol. 2009;24(10):597–602. 10.1007/s10654-009-9377-0 [DOI] [PubMed] [Google Scholar]
- 31. Steinbach R, Perkins C, Tompson L, et al. : The effect of reduced street lighting on road casualties and crime in England and Wales: controlled interrupted time series analysis. J Epidemiol Community Health. 2015;69(11):1118–24. 10.1136/jech-2015-206012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linden A: Conducting interrupted time-series analysis for single-and multiple-group comparisons. Stata J. 2015;15(2):480–500. 10.1177/1536867X1501500208 [DOI] [Google Scholar]
- 33. Lagarde M: How to do (or not to do) … Assessing the impact of a policy change with routine longitudinal data. Health Policy Plan. 2012;27(1):76–83. 10.1093/heapol/czr004 [DOI] [PubMed] [Google Scholar]
- 34. E Clapham H, A Wills B: Implementing a dengue vaccination programme-who, where and how? Trans R Soc Trop Med Hyg. 2018;112(8):367–8. 10.1093/trstmh/try070 [DOI] [PMC free article] [PubMed] [Google Scholar]


