Figure 2.
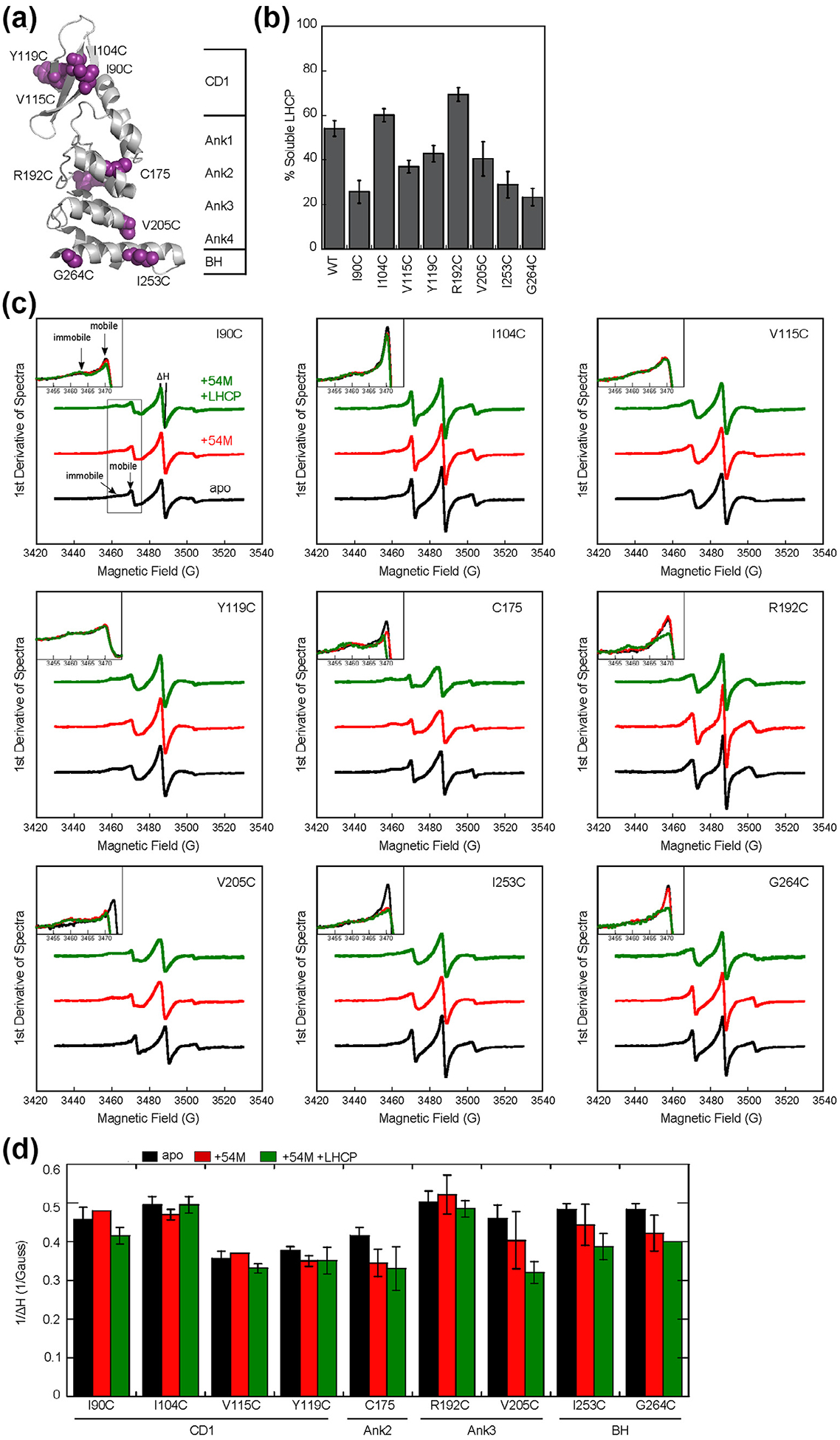
The cpSRP54 M-domain and substrate induce immobilization of spin probes across the ankyrin repeat domain and the BH of cpSRP43. (a) Structure of the cpSRP43 SBD highlighting residues used for SDSL-EPR analyses. (b) The chaperone activities of single cysteine cpSRP43 mutants were analyzed using the turbidity assay. Reactions used 1 μM LHCP and 2.5 μM WT or mutant cpSRP43. All comparisons were relative to cysteineless cpSRP43(C175A, C297S), which is denoted as WT. (c) EPR spectra for spin labels at indicated positions in apo-cpSRP43 (black), cpSRP43 bound to 54 M (red), and cpSRP43 bound with both 54 M and LHCP (green). The insets show a zoom-in of the spectra at 3450–3475 G, which contain the peaks reporting on the mobile and immobile populations of the spin probe. (D) Summary of the effects of 54 M and LHCP on the central line width (ΔH0) of the spectra for spin labels at various positions in the SBD, quantified from the data in part C. All values are reported as mean ± S.D., with n ≥ 2.
