Figure 6.
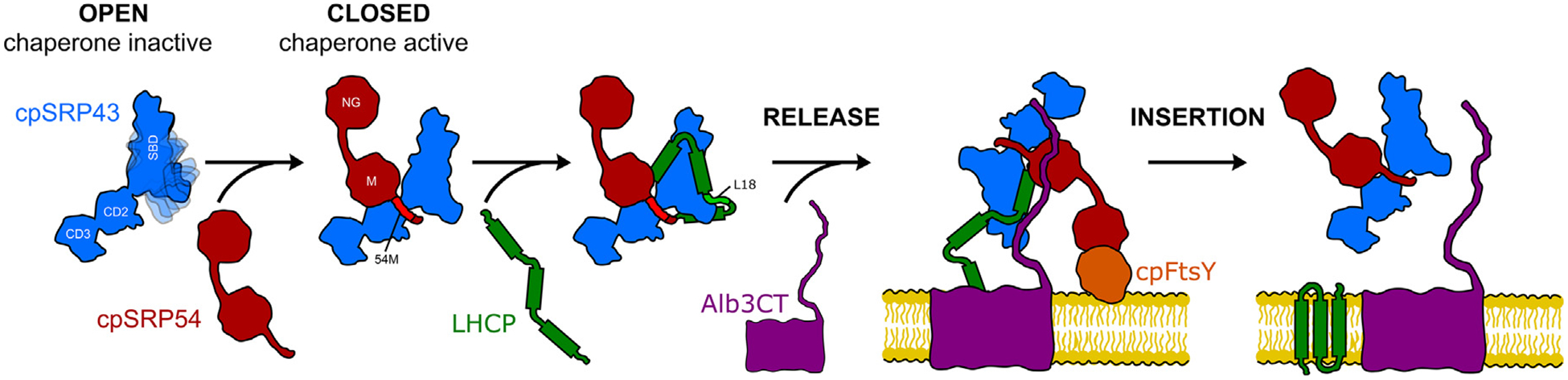
Model for allosteric regulation of cpSRP43’s chaperone activity. In the open conformation, the SBD’s ankyrin repeat domain and BH are flexible and disordered, resulting in an inactive chaperone. The interaction of cpSRP43 with the cpSRP54 M-domain or with the L18 motif on the LHCP substrate stabilizes the closed conformation, in which the Ankyrin repeat domain is more stably folded. This rearrangement enables the formation of contiguous contact surfaces on the cpSRP43-SBD for the substrate TMDs and thus high chaperone activity. The interaction of cpSRP54 with cpFtsY delivers the transit complex to the Alb3 translocase at the thylakoid membrane, where Alb3 triggers cpSRP43 to release LHCP and mediates its insertion into the membrane.
