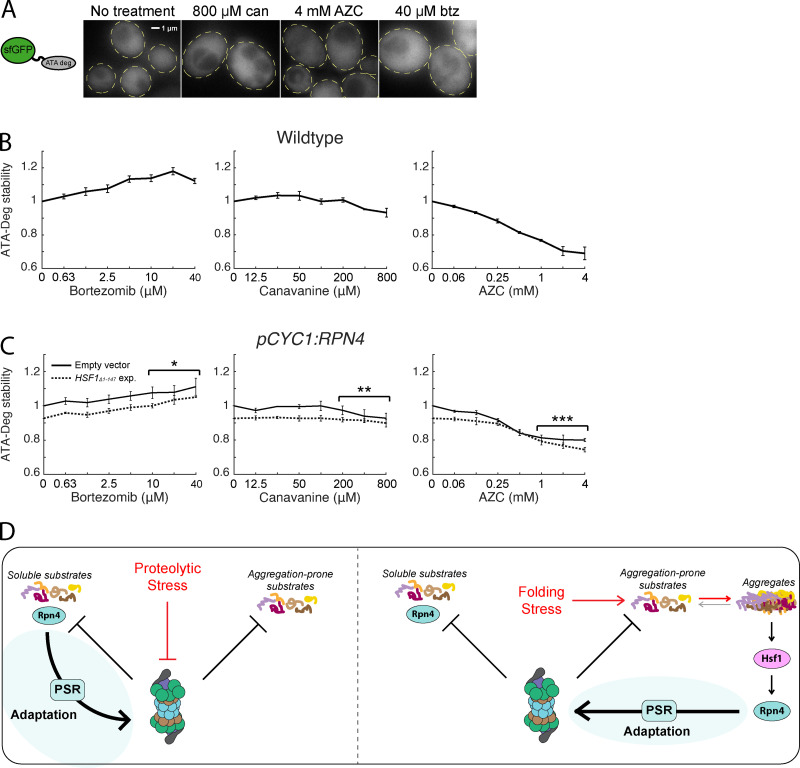
Figure 6.
Folding stressors do not impair degradation of soluble UPS substrates. (A) Fluorescent localization of ATA-Deg (GFP) in cells treated with 800 µM canavanine, 4 mM AZC, 40 µM bortezomib, or no treatment for 5 h. Cells are outlined in yellow dashed lines. (B) Measurements of fold ATA-Deg stability in titrations of bortezomib, canavanine, or AZC. (C) Fold ATA-Deg stability in drug titrations in cells expressing an empty vector (solid) or a copy of HSF1Δ1-147 (dotted). Significance was collectively tested across the three highest concentrations measured by combining data from these concentrations into one group per genetic context, then performing a paired two-sided Student’s t test. Error bars throughout denote standard error for n ≥ 3 biological replicates. (D) Model for UPS adaptation by the PSR to proteolytic and folding stressors. Left: Proteolytic stressors increase the stability of all proteasome substrates. This includes Rpn4, whose accumulation leads to PSR activation. Right: Folding stressors causes some proteasome substrates to sequester into aggregates. Aggregation triggers the HSR, activates transcription of RPN4, and causes PSR activation. *, P < 0.05; **, P < 0.01; and ***, P < 0.001. btz, bortezomib; can, canavanine; exp., exposure.