FIGURE 2.
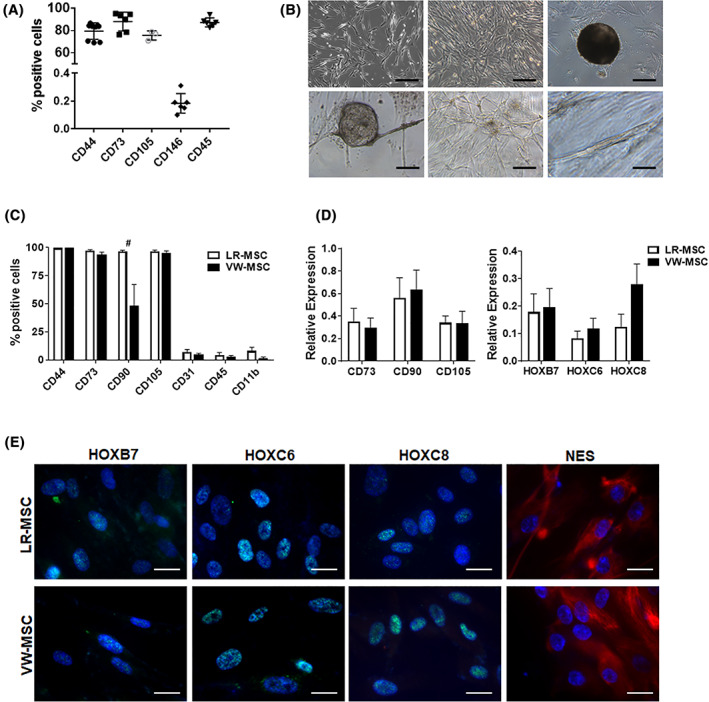
Isolation and characterization of lung‐resident mesenchymal stem cells (LR‐MSCs). A, Normal lung tissue was homogenized by collagenase digestion and the crude cell extract was analyzed by flow cytometry using the indicated (MSC) markers. Data (column scatter plots) include the mean ±SD, n = 4‐7. B, Representative phase contrast micrographs of cells 10 to 12 days after initial plating showed typical mesenchymal (flattened and fibroblast‐like) cell morphology. Cultivated LR‐MSCs form clonally cell aggregates upon prolonged culturing (CFU, colony‐forming units). When LR‐MSCs were embedded in GFR‐Matrigel as 3D‐spheroids, VW‐MSC‐typical in‐gel sprouting and Matrigel invasion (tube formation) was observed. Scale bar 50 μm. C, FACS analysis of cultured LR‐MSCs show that LR‐MSCs are positive for CD90, CD73, CD105, and CD44 but negative for lineage cell markers CD45, CD31, and CD11b indicating no considerable contamination by other types of progenitors. FACS data summarizing for at least four independent experiments (±SEM) are shown. Ex vivo isolated hITA (human internal thoracic artery)‐derived VW‐MSCs were shown as “control.” D, Relative amounts of transcripts of the indicated genes including the VW‐MSC‐specific “HOX code” were further determined by qRT‐PCR in LR‐MSCs and compared to VW‐MSCs (biological replicates: n = 5‐7 per group and gene). Relative transcript levels of analyzed genes were normalized to beta‐actin mRNA (set as 1). E, LR‐MSCs and VW‐MSCs were seeded on gelatin‐coated cover‐slips and HOXB7, HOXC6, HOXC8, and NES expression were detected by immunofluorescence using confocal microscopy (blue, DAPI). Representative photographs from four independent experiments are shown. Scale bar indicates 10 μm
