FIGURE 7.
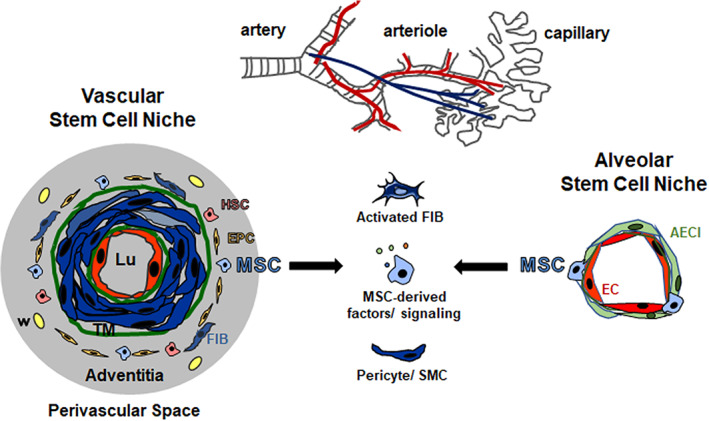
Lung‐resident MSCs: Localization and potential contributions to lung cancer. Vascular wall‐resident mesenchymal stem cells (VW‐MSCs; blue) are localized in the prominent adventitial stem cells niche, the so‐called vasculogenic zone of large and mid‐sized arteries (left side). The vasculogenic zone is a vascular mural zone located within the adventitia and close to the tunica media (TM). The border between media and adventitia is marked by outer elastic membrane (green). This vascular stem cell niche further harbors endothelial progenitor cells (EPC) and hematopoietic stem cells (HSC). Within lung tissue, VW‐MSCs could most likely be designated as LR‐MSCs, as LR‐MSCs were not only lung‐resident but also tissue‐specific and in particular showed tissue specificity of the vessel wall. The alveolar stem cell niche (right side) combines the vascular niche with the airway niche. Within the smallest blood vessels (capillaries) LR‐MSCs can be found in the small alveolar interstitium, close to the capillary endothelial cells that share their intermediate basement membrane with sheet‐like alveolar epithelial cells (type I, AECI). LR‐MSCs contribute to the maintenance of tissue integrity in adult lungs. Following a pathological trigger, however, lung injury might at least be partially mediated by endogenous MSCs. Due to their vascular localization, lung‐resident MSCs could be the first line cells, which are available on the basis of their anatomic location as first point of contact for tumor cells and secreted factors. Mobilized and/or activated MSCs bear the ability to migrate toward primary tumors and metastatic sites, thereby impacting on lung cancer progression, either by an altered tumor‐promoting secretory profile, by differentiating into pericytes and smooth muscle cells (SMCs) and/or into activated fibroblasts (myofibroblasts and cancer‐associated fibroblasts). Moreover, upon lung damage, tissue‐resident MSCs could be one cell of origin for cancer development or progression. Finally, in terms of manufacturing exogenous MSCs with superior repair capabilities, lung MSCs itself might be logically more efficient for a therapeutically approach than MSCs derived from other tissues for the treatment of lung diseases
