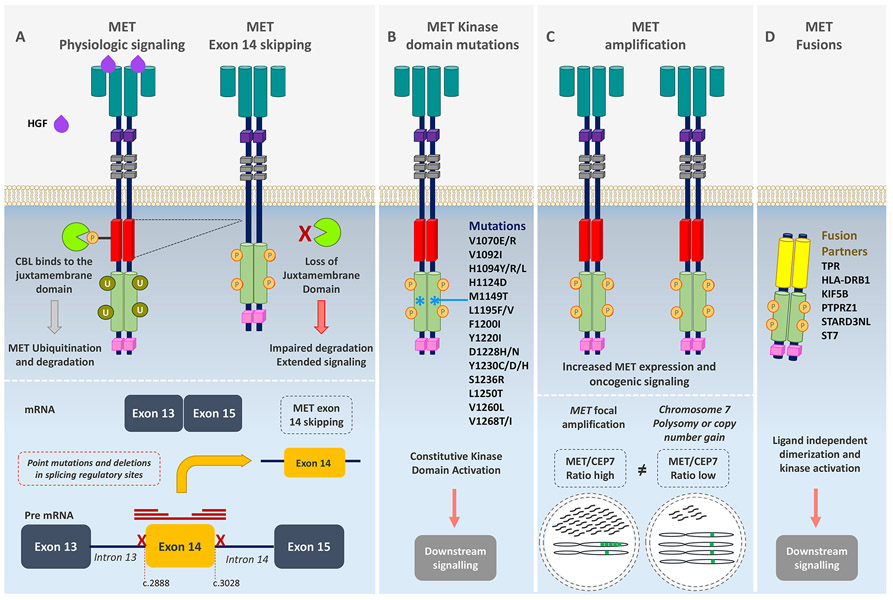
Figure 1. Mechanisms of MET oncogenic activation.
(A) MET activation is initiated after ligand binding of the hepatocyte growth factor (HGF) to the SEMA domain to the extracellular portion of the MET receptor, inducing receptor dimerization and phosphorylation of the intracellular domain leading to downstream signaling of several pathways. The E3 ubiquitin ligase CBL binds to reside Y1003 in the juxtamembrane domain (encoded in part by exon 14), resulting in receptor ubiquitination and degradation. MET exon 14 alterations including mutations or deletions in splicing regulatory sites, leading to exon skipping, deletion of a portion of the juxtamembrane domain, impaired CBL binding, decreased MET turnover, and ongoing oncogenic downstream pathway signaling. (B) Point mutations in the tyrosine kinase domain result in ligand-independent MET activation through autophosphorylation of the tyrosine kinase domain, conveying sustained oncogenic downstream pathway signaling. (C) Focal MET amplification leads to higher levels of MET transcription and MET expression. MET amplification (high MET/CEP7 ratio) differs from chromosome 7 polysomy or copy number gain in which the entire chromosome is duplicated (low MET/CEP7 ratio). Focal MET amplification and, consequently high MET expression, leads to enhanced ligand-independent oncogenic signaling by receptor auto-dimerization or oligomerization and auto-phosphorylation. (D) Gene rearrangements involving the MET tyrosine kinase domain result in fusion proteins that typically self-dimerize in a ligand-independent manner, leading to phosphorylation of the tyrosine kinase domain.