FIGURE 2.
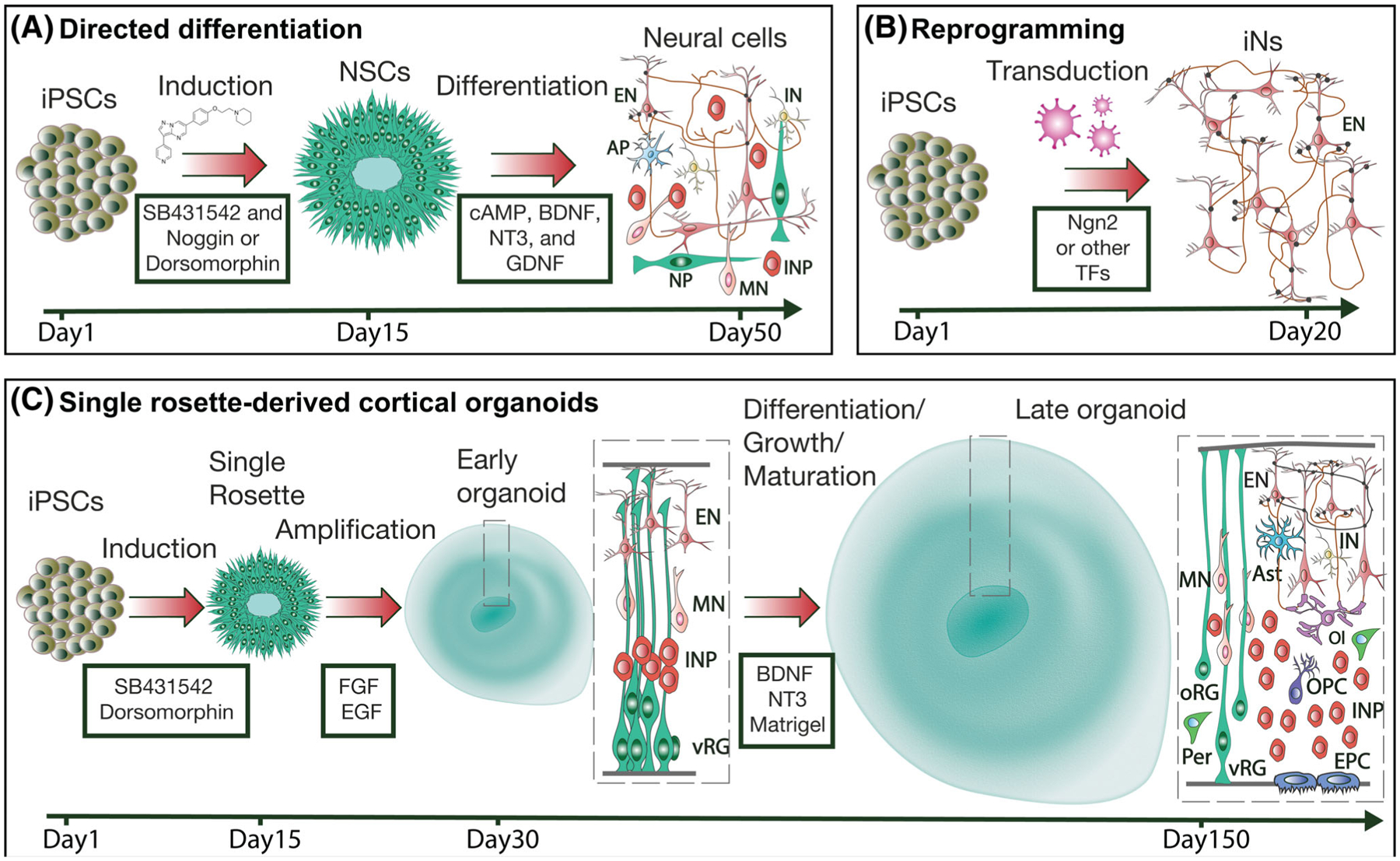
Stem cell-based tool box for modeling human cortical development and disorders. A, Typical protocol for directed neural differentiation of human pluripotent stem cells. During the neural induction step, stem cells are converted into neuroepithelial cells/neural stem cells. A dual SMAD inhibition protocol is often used for this step.58 These cells are typically grown in clusters of neural rosettes. During differentiation, neural stem cells are exposed to trophic factors, such as cAMP, BDNF, NT3, and GDNF, which promote neuronal differentiation, maturation, and survival. After 50 days of differentiation, the cultures typically consist of a mixed population of cells, including neural progenitors (NP), intermediate neural progenitors (INP), migrating neurons (MN), astrocyte progenitors (AP), excitatory neurons (EN), and inhibitory neurons (IN). B, Typical protocol for reprogramming of human pluripotent stem cells into induced neurons (iN). Stem cells are transduced with lentiviruses carrying specific transcription factor(s) and an antibiotic selection cassette required for efficient conversion and selection. A TET-ON/OFF system is typically used to control the level and timing of expression. For example, a relatively uniform population of functionally mature excitatory neurons can be produced via Ngn2-mediated reprogramming. C, Protocol for generating cortical organoids. We propose that cortical organoids can be generated from single neural rosettes to improve organoid-to-organoid reproducibility and facilitate reliable identification of cellular and molecular deficits in ASDs. The protocol will consist of a neural induction step for the production of neural rosettes that can be isolated and propagated in suspension culture. Initially, isolated single rosettes will be grown in the presence of growth factors to promote neural stem cell proliferation. Later, single rosette-derived organoids will be imbedded in Matrigel (or another scaffolding material) and cultured in the presence of trophic factors, such as BDNF and NT3, to promote differentiation, growth, and maturation. In contrast to 2D protocols, a diversity of properly organized neural cells can be generated in organoids at different developmental stages, including ventricular and outer radial glia (vRG and oRG, respectively); intermediate progenitors (INP); migrating, neurons (MN); excitatory and inhibitory neurons (EN and IN, respectively); astrocytes (Ast); oligodendrocytes and oligodendrocyte progenitors (Ol and OPC, respectively); pericytes (Per); and ependymal cells (EPC)
