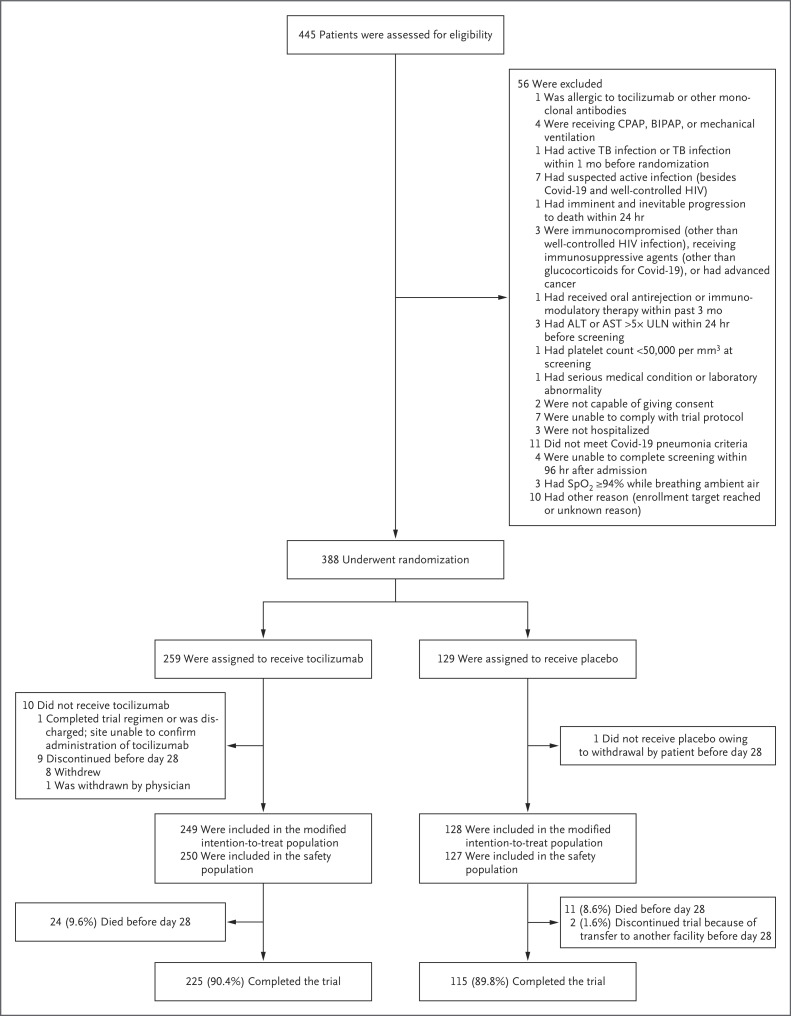
Figure 1. Enrollment, Randomization, and Follow-up.
The modified intention-to-treat population included all the patients who underwent randomization and received tocilizumab or placebo. Patients may have been excluded for more than one reason. A total of 389 patients underwent randomization, and 388 patients had data that could be evaluated. (One patient underwent randomization before local institutional review board approval of the trial site. This patient did not receive tocilizumab or placebo, and no further data were collected.) One patient who was randomly assigned to the placebo group received tocilizumab and was included in the tocilizumab group in the safety population. The 2 patients who had another reason for discontinuation of placebo were transferred to other facilities. Patients who completed day 28 of the trial before discharge or were discharged before day 28 were considered to have completed the trial. Percentages shown are for the modified intention-to-treat population. ALT denotes alanine aminotransferase, AST aspartate aminotransferase, BIPAP bilevel positive airway pressure, CPAP continuous positive airway pressure, HIV human immunodeficiency virus, SpO2 oxygen saturation as measured by pulse oximetry, TB tuberculosis, and ULN the upper limit of the normal range.