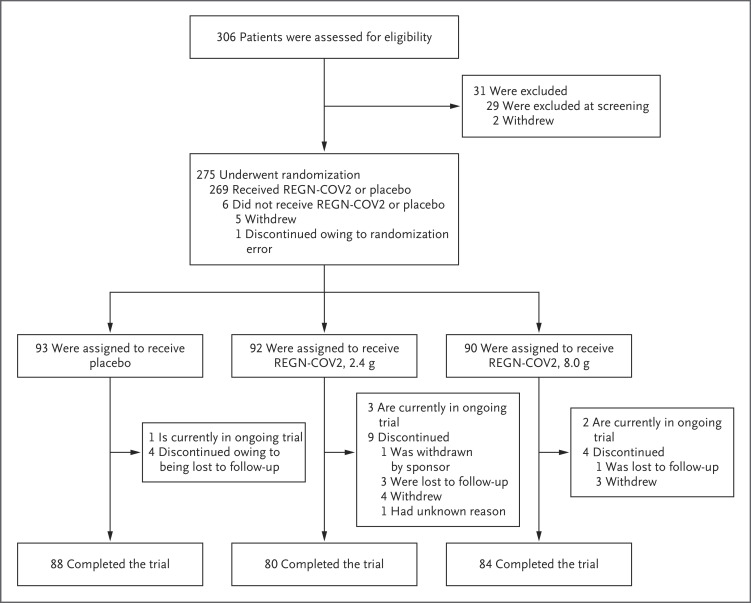
Figure 1. Screening, Randomization, and Treatment.
One patient underwent randomization in error, and Regeneron requested that the patient withdraw from the trial. Four patients in the low-dose REGN-COV2 group withdrew consent: one patient could not participate in the follow-up period, one patient could not have blood drawn and an intravenous line placed, and two patients withdrew consent with no additional information available. Three patients in the high-dose REGN-COV2 group withdrew consent: one patient could not participate in the follow-up period, one patient could not have blood drawn and an intravenous line placed, and one withdrew consent with no additional information available.