Figure 5:
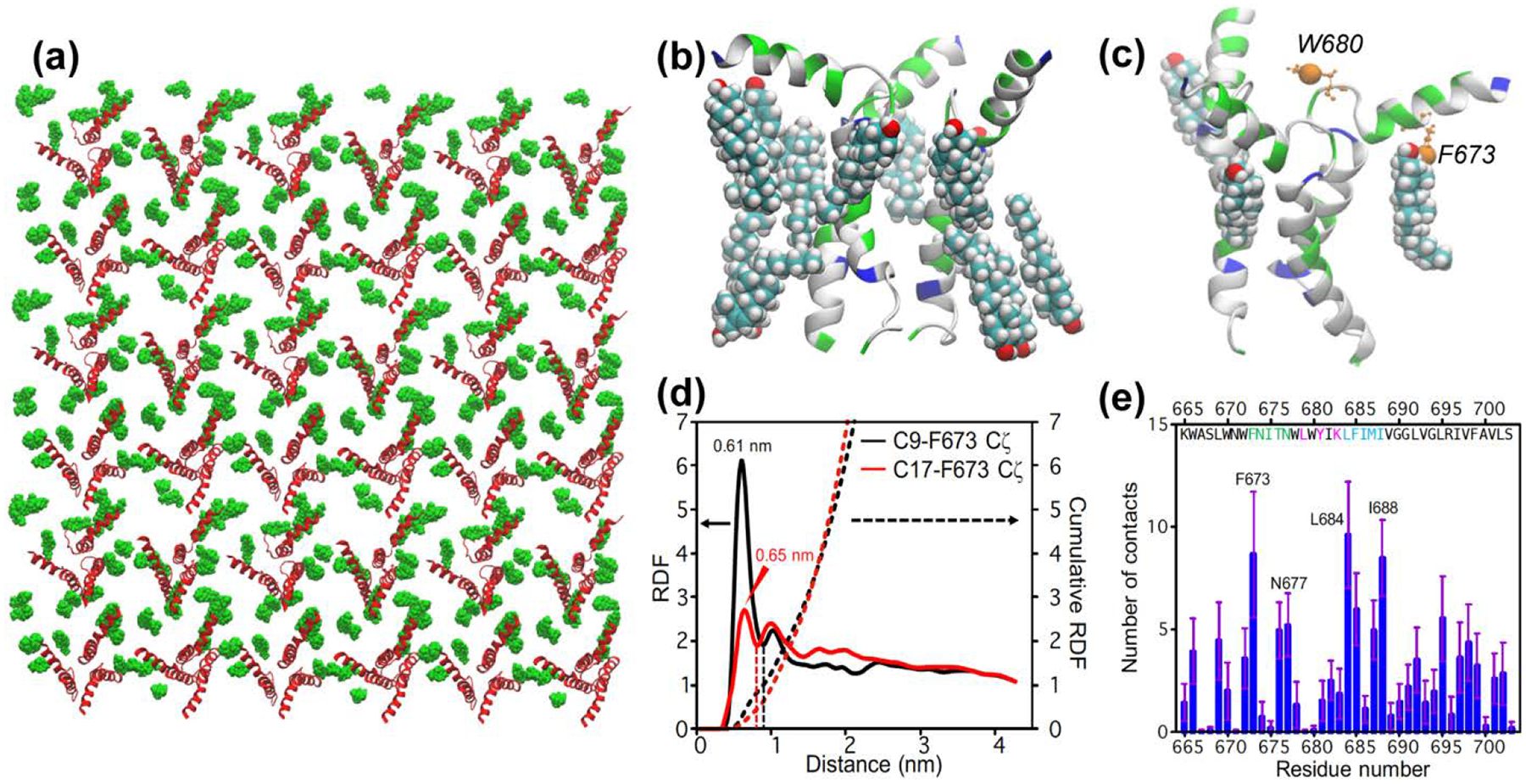
All-atom molecular dynamics simulation of CHOL-gp41 interactions at P : L : C = 1 : 16 : 7. (a) Top view of the equilibrated lipid membrane, showing protein (red) and cholesterol (green) molecules in the upper leaflet. (b) A representative structure showing cholesterol molecules within 3 Å of a trimer. (c) A representative snapshot showing the positions of F673 Cζ and W680 Cζ3 (orange spheres) in the MPER-TMD trimer. Three bound cholesterols whose C9 atoms are within 7.0 Å from F673 Cζ are shown. These sub-nanometer contacts can be detected by 13C-19F REDOR dipolar coupling measurements. (d) Radial distribution function (RDF) and cumulative sums (dotted lines) of cholesterol C9 and C17 from F673 Cζ. The value of the cumulative RDF up to the first minimum (dashed lines) for C9 and C17 is averaged to give the number of bound cholesterols per gp41 monomer, which is 0.75. Thus, 2.3 cholesterols are bound near the MPER per trimer. (e) Number of cholesterol atoms in close contact with each MPER-TMD residue.
