Extended Data Fig. 3. FMC63–28Z neurologic toxicities.
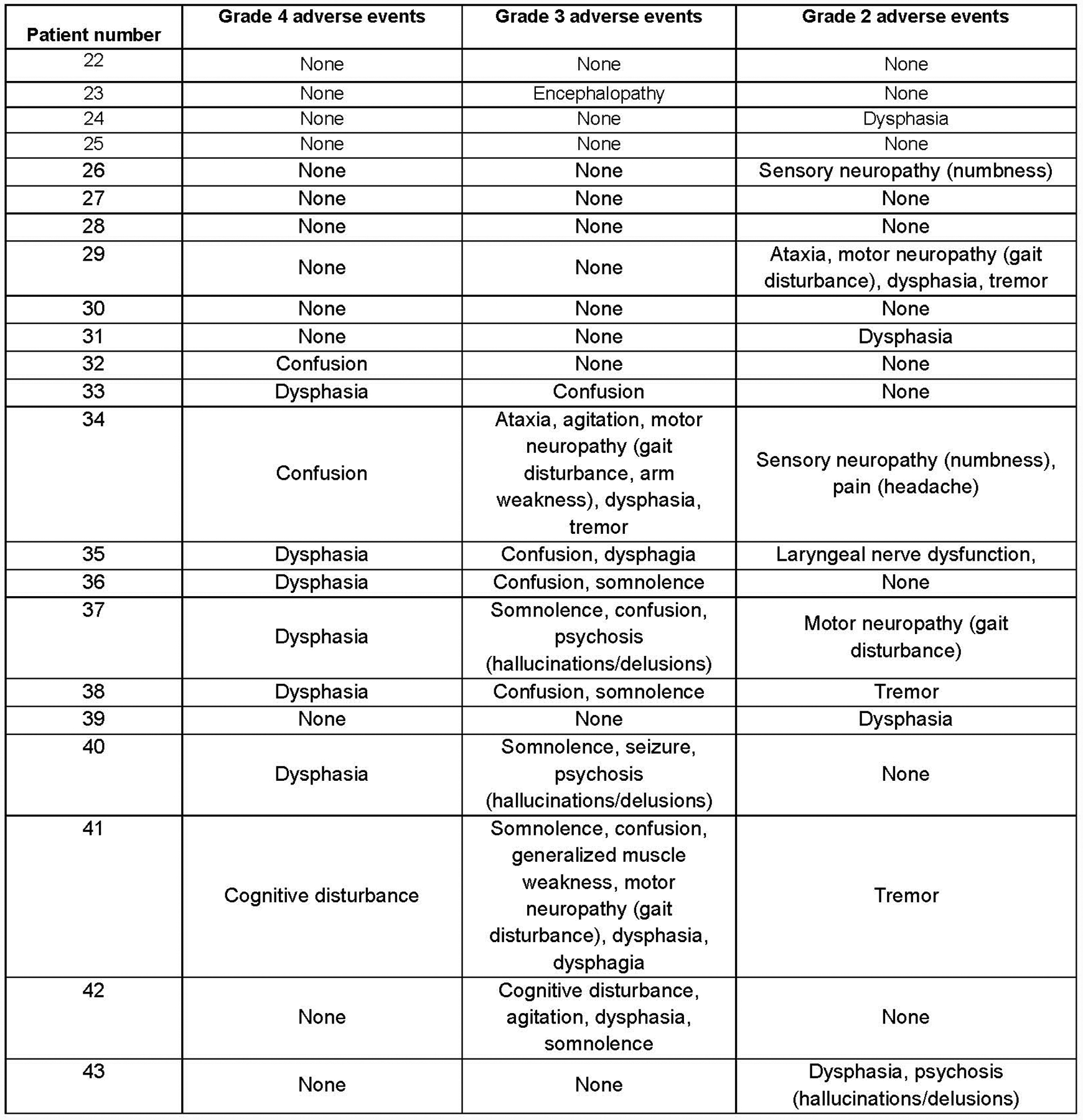
Neurologic toxicity with FMC63–28Z. All grade 4, 3, and 2 neurologic adverse events within the first month after CAR T-cell infusion are listed. Grading by National Cancer Institute Common Terminology Criteria for Adverse Events Version 3; all adverse events listed under “Neurologic” are included except syncope. Syncope was not included because it was associated with hypotension from cytokine-release syndrome. The highest grade of each adverse event experienced by each patient is listed. For example, if a patient had both Grade 2 and Grade 3 confusion at different times, confusion is only listed under Grade 3.
