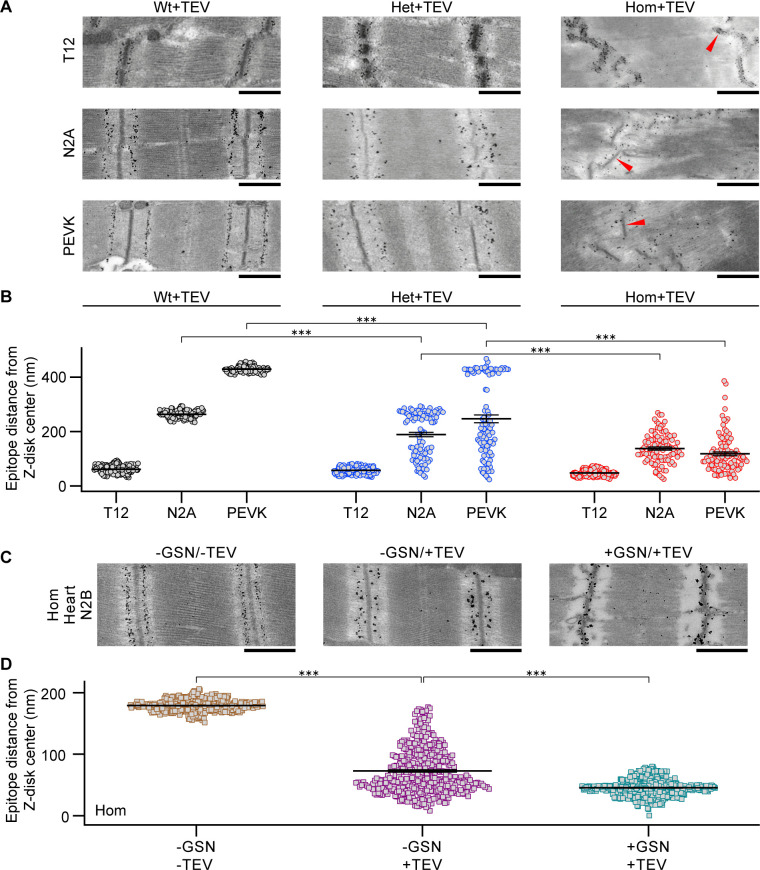
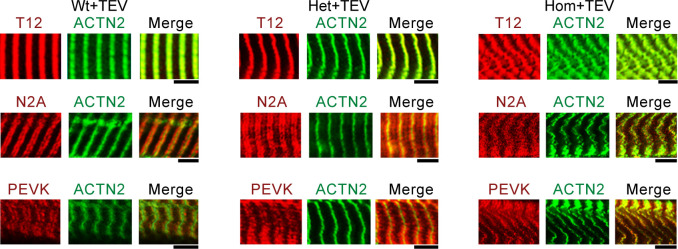
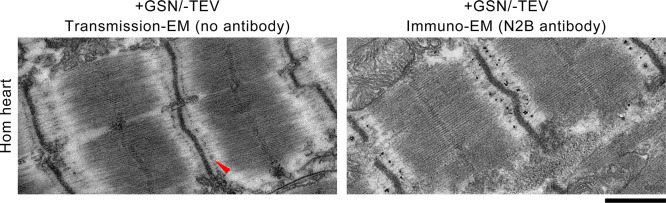
Figure 5. Incomplete elastic recoil of cleaved titin to the Z-disk.
(A) Nanogold immunoelectron micrographs of skeletal fibers labeled with different antibodies to I-band titin (T12, N2A, and proline-glutamate-valine-lysine-rich region (PEVK)). Shown are examples of Wt, Het, and Hom fibers after TEV protease treatment, held passively for 30 min at a stretched length. Red arrows point to Z-disks. (B) Recoil of elastic titin to the Z-disk in skeletal fibers quantified by measuring the antibody-epitope to Z-disk-center distance (n = 100 measurements/condition; SL range, 2.9–3.0 µm). (C) Passively fixed Hom TC cardiomyocytes labeled for the N2B titin element (central I-band) showing regular staining in controls (−GSN/−TEV; left). Titin cleavage by TEV protease (−GSN/+TEV, middle) or actin-removal by gelsolin treatment, followed by TEV treatment (+GSN/+TEV), caused partial (middle) or full (right) recoil of titin springs to Z-disks. (D) Quantification of titin recoil in Hom TC cardiomyocytes from images as in (C); measurement as in (B) (n = 200 measurements/group, evenly from 10 sarcomeres/group; SL range, 2.2–2.3 µm). Stats: ANOVAs with Tukey’s HSD post hoc procedure, further confirmed via ranked sum assessment (analysis not shown). Note: error bars are very small.