Figure 4.
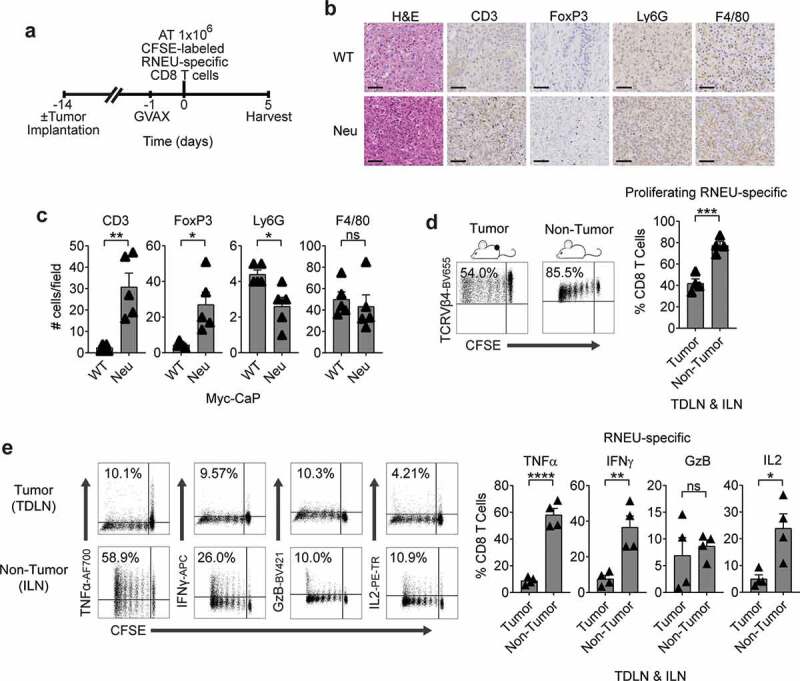
Myc-CaP/Neu Tumors Attenuate The RNEU-specific CD8 T Cell Response Induced by Vaccination with Her-2/neu Expressing Cells.
(a) Treatment scheme for tumor implantation with 1 × 106 Myc-CaP/Neu cells. One day after the neu-expressing (GM-CSF–secreting) vaccine (GVAX) was administrated, 1 × 106 high-avidity CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells were adoptively transferred (AT) into the mice. On day 5, tumor-draining lymph nodes (TDLNs; tumor) and inguinal LNs (ILNs; non-tumor) were harvested and analyzed by flow cytometry. (b) Tumor-infiltrating lymphocytes (TIL; CD3), regulatory T cells (FoxP3), myeloid-derived suppressor cells (Ly6 G), and macrophages (F4/80) of indicated murine allografts (representative immunohistochemistry; repeated x 2). Scale bar = 50 μm. (c) Counts of immune cells in tumor microenvironment (TME). (d) Percentages of proliferating TCRVβ4+ CD8 T cells in TDLNs and ILNs (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). (e) Percentages of cytokine production by RNEU-specific TCRVβ4+ CD8 T cells in TDLNs and ILNs (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). Proliferating TCRVβ4+ CD8 T cells, and their cytokine production, were calculated as fraction of TCRVβ4+ CD8 T cells. ILNs and TDLNs were isolated from naïve and tumor-bearing mice, respectively. Whole-cell vaccination (GVAX) was prepared as described in materials and methods.
