Fig. 5.
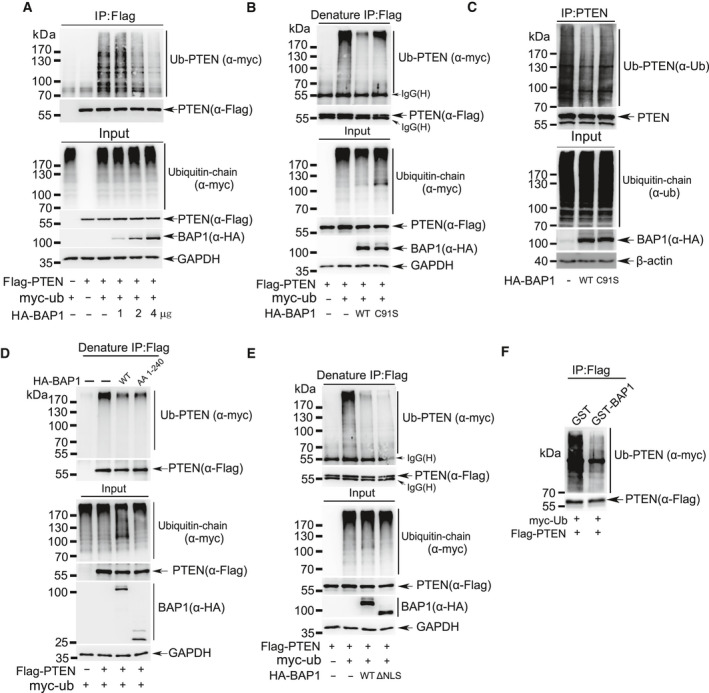
BAP1 deubiquitinates PTEN. (A) Lysates from 293T cells transfected with Flag‐PTEN, Myc‐Ubiquitin together with an increasing amount of BAP1 were immunoprecipitated with anti‐Flag antibody, and followed by western blotting analysis with anti‐Myc antibody. (B) Lysates from 293T cells transfected with Flag‐PTEN, Myc‐Ubiquitin together with BAP1WT or mutant BAP1C91S were immunoprecipitated with anti‐Flag antibody under denaturing condition, and followed by western blotting analysis with anti‐Myc antibody. (C) Lysates from HeLa cells transfected with wild‐type BAP1 or mutant BAP1C91S were immunoprecipitated with anti‐PTEN antibody, and followed by western blotting analysis with anti‐ubiquitin antibody. (D‐E) Lysates from 293T cells transfected with Flag‐PTEN, Myc‐Ubiquitin together with full‐length HA‐BAP1, truncated HA‐BAP11‐240 (D) or HA‐BAP1ΔNLS (E) were immunoprecipitated with anti‐Flag antibody under denaturing condition, and followed by western blotting analysis with anti‐Myc antibody. (F) Ubiquitinated PTEN was purified from 293T cells transfected with Flag‐PTEN and Myc‐Ubiquitin, and then incubated with purified GST‐BAP1 from E. coli. PTEN ubiquitination level was detected by western blotting with anti‐Myc antibody. All above experiments were repeated at least 3 times.
