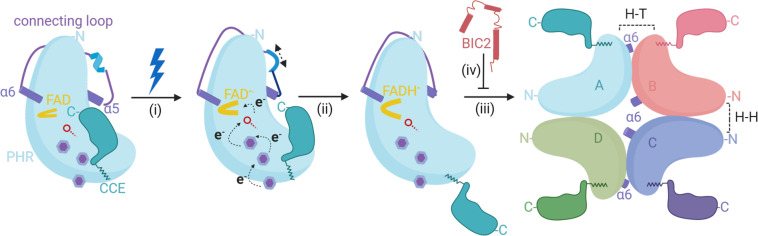
Fig. 6. Proposed model of blue light-induced photoactivation and inactivation of plant CRYs.
CRYs exist as a monomer non-covalently linked to the oxidized FAD with the CCE tail bound to the PHR domain. The dynamic interconnecting loop in the inactive monomeric state is highlighted in purple with a short α-helix in light blue. (i) Blue light illumination causes the FAD to absorb energy and accept electrons via the tryptophan triad. The purple benzene rings represent the tryptophan residues and the red line with an open circle represents amino acid D393. After accepting the electrons, the FAD becomes FAD•− and adopts a U-shaped conformation followed by an overall change in the conformation of CRY including alterations in the interconnecting loop. (ii) The FAD•− becomes neutralized by accepting one proton from D393 and becomes FADH•. The overall change in the conformation of the CRY structure induced by photoactivation leads to the loss of secondary structural elements in the interconnecting loop. (iii) The photoactivated CRYs adopts an open conformation with CCE tail projecting outward leading to formation of homo-tetramer. The formation of the oligomeric state is guided by the dynamic interconnecting loop that allows the movement of residues in α-6 to participate in forming active interface in the oligomeric state. (iv) The photoactivated CRYs are readily inhibited by the presence of BICs which disrupts or prevents the H–T interface in the homo-tetramer.