Abstract
A potentially important aspect in the regulation of tumour metastasis is endocytosis. This process consists of internalisation of cell-surface receptors via pinocytosis, phagocytosis or receptor-mediated endocytosis, the latter of which includes clathrin-, caveolae- and non-clathrin or caveolae-mediated mechanisms. Endocytosis then progresses through several intracellular compartments for sorting and routing of cargo, ending in lysosomal degradation, recycling back to the cell surface or secretion. Multiple endocytic proteins are dysregulated in cancer and regulate tumour metastasis, particularly migration and invasion. Importantly, four metastasis suppressor genes function in part by regulating endocytosis, namely, the NME, KAI, MTSS1 and KISS1 pathways. Data on metastasis suppressors identify a new point of dysregulation operative in tumour metastasis, alterations in signalling through endocytosis. This review will focus on the multicomponent process of endocytosis affecting different steps of metastasis and how metastatic-suppressor genes use endocytosis to suppress metastasis.
Subject terms: Metastasis, Breast cancer, Cell invasion, Bone metastases
Background
Cancer is the second leading cause of global mortality.1 The spread of cancer cells from the primary tumour to distant organs and their subsequent progressive colonisation is referred to as metastasis. It is estimated that 90% of cancer-related deaths are due to metastatic disease rather than to the primary tumour growth. Typically, treatments for metastatic cancer are systemic therapy involving chemotherapy or molecular drugs, hormonal agents, immune checkpoint drugs, radiation therapy or surgery. Despite progress in extending cancer-survivorship rates,2 limited progress has been made in the treatment of metastatic cancer due to its complex nature and an inadequate understanding of the molecular and biochemical mechanisms involved.
Metastasis is a multistep process involving tumour cell invasion to neighbouring areas, intravasation into the bloodstream, arrest in the capillary bed of a secondary organ, extravasation from the circulatory system and colonisation at the secondary site.3 All of the above steps occur via complex interactions between cancer cells and their microenvironments. Despite the documented complexity and redundancy of the metastatic process, mutation or changes in the expression of single genes have been reported to alter metastatic ability. Genes that are involved in the promotion of metastasis at distant sites are referred to as metastasis promoting genes. Expression of these genes facilitates cancer cell establishment of appropriate interactions with changing microenvironments to promote continued survival and proliferation at secondary sites. Similarly, genes that inhibit the process of metastasis without affecting the growth of the primary tumour are referred to as metastasis suppressor genes and are described in detail in the later part of this review.
This review will highlight an often-overlooked aspect of metastasis, receptor endocytic pathways. Contributing to each step in metastasis is the distribution of multiple cell-surface receptors on tumour and microenvironmental cells. Receptor signalling is, in turn, modulated by endocytosis (internalisation, recycling or degradation). In recent years, there has been significant progress made towards understanding the mechanisms of the endocytosis pathway and its alterations that occur during metastasis. A growing body of literature suggests that receptor endocytosis affects metastasis and could be a tool for the functioning of metastasis suppressor or metastasis promoters. This review will focus on the role of endocytosis in metastasis and how these pathways are used by metastasis suppressors.
Endocytic pathways and metastasis
The term ‘endocytosis’ is derived from the Greek word ‘endon’, meaning within, ‘kytos’, meaning cell and ‘-osis’, meaning process. So, endocytosis is the process by which cells actively internalise molecules and surface proteins via an endocytic vesicle. Depending on the cargo type, internalisation route and scission mechanism, there are three general modes of vesicular endocytic trafficking that coexist in the cell and operate concurrently: phagocytosis, pinocytosis and receptor-mediated endocytosis. In phagocytosis, the cell’s plasma membrane surrounds a macromolecule (large solid particles > 0.5 μm) or even an entire cell from the extracellular environment and generates intracellular vesicles called phagosomes.4 Cellular pinocytosis/cellular drinking is a process in which fluids and nutrients are ingested by the cell, by pinching in and forming vesicles that are smaller than the phagosomes (0.5–5 μm).5 Both phagocytosis and pinocytosis are non-selective modes of taking up molecules. However, there are times when specific molecules are required by cells and are taken up more efficiently by the process of receptor-mediated endocytosis (RME). The endocytosis of specific cargoes via specific receptors can take place by clathrin-mediated (CME), caveolae-mediated (CavME), clathrin- and caveolae-independent endocytic (CLIC/GEEC) pathways. These endocytic pathways are briefly described below. Table 1 links selected endocytic proteins to in vitro components of the metastatic process and in vivo metastasis in cancer.
Table 1.
Validated roles of endocytic proteins in metastasis.
| Endocytic protein | Function(s) | Phenotypic effects (in vitro, unless noted) | Cancer types studied |
|---|---|---|---|
| Clathrin-mediated endocytosis (CME) | |||
| AP2 | Recruits cargo and clathrin to growing clathrin coated pits | Modulates tumour cell migration, invasion and chemotaxis through CXCR-2. | Ovarian, pancreatic and melanoma cancer89 |
| Clathrin | Component of the coat protein for membrane invagination in endocytosis | Clathrin light chain isoform (CLCb) is upregulated and associated with poor prognosis. NSCLC cells expressing CLCb exhibit increased cell migration and the metastasis (in vivo). | NSCLC17 |
| Dynamin | Dynamins are large GTPase, encoded by three genes in mammals, required for scission of newly formed vesicles from the membrane | Dynamin 1 and 2 are known to enhance cancer cell proliferation, tumour invasion and metastasis, whereas Dynamin 3 has a tumour-suppressive role. | NSCLC cells90 |
| Dynamin 2 overexpression correlates with poor prognosis. | Prostate cancer18 | ||
| Caveolin-mediated endocytosis (CavME) | |||
| Caveolin | Major coat protein of caveolae and involved in invagination of lipid raft domain | In early stages of the disease caveolin functions predominantly as a tumour suppressor, whereas at later stages its expression is associated with tumour progression and metastasis. The late-stage tumour progression and metastasis effect of CAV-1 has been attributed to tyrosine (Tyr14) phosphorylation of its protein product by Src kinases. | Hepatomas, ovarian cancers, prostate cancer and breast cancer30,31 |
| CAV-1 is often deleted in human cancers and acts by inhibiting cytokine receptor signalling. | Breast cancer31,32 | ||
| Knockdown of CAV-1 reduced velocity, directionality and persistency of cellular migration. | Breast and prostate cancers31,32 | ||
| Positive expression of CAV-1 is a marker of histopathological grade and poor prognosis (pancreatic cancer). Low expression of CAV-1 is associated with poor prognosis (hepatic cancer). | Pancreatic adenocarcinoma and lung cancer; hepatocellular carcinoma33 | ||
| Clathrin-independent endocytosis (CIE) | The endocytic vesicles involved in CIE have no distinct coat | CIE pathway suppresses cancer cell blebbing and invasion through GTPase-activating protein GRAF1. | Colon cancer39 |
| Endosomal trafficking proteins | |||
| ARF subfamily: | Small GTPase family | ||
| ARF1 | Regulates assembly of different types of ‘coat’ complexes onto budding vesicles, involved in secretory pathway and activates lipid-modifying enzymes | Controls cellular migration and proliferation by regulating interaction between β1-integrin and key proteins of focal adhesions, such as paxillin, talin and FAK. | Breast cancer91,92 |
| ARF4 | Regulates retrograde transport from endosomes to the TGN | Tensin-mediated cellular invasion and migration is modulated by ARF4-dependent internalisation of α5β1-integrins to late endosomes/lysosomes and consequently their degradation. | Ovarian cancer93 |
| ARF6 | Regulates endocytic membrane trafficking and actin remodelling | ARF6 promotes E-cadherin internalisation and facilitates disassembly of adherens junction for cellular motility and invasion. | MDCK cells,94,95 glioma, breast cancer96 |
| ARF6 inhibitor impairs melanoma pulmonary metastasis. | Melanoma97 | ||
| Ras-homologue (RHO) subfamily: | |||
| RHOA | Facilitates the assembly of contractile actomyosin filaments in focal adhesion complexes and vesicle trafficking | Loss of RHOA expression prevents the endocytosis of multiple receptors and enhances breast cancer metastasis in vivo with a concomitant increase in CCR5 and CXCR4 chemokine signalling. | Breast cancer98 |
| N-WASP regulates endosomal recycling of LPAR1 which increases RhoA-mediated contractile responses, cell steering and spontaneous metastasis. Mice with N-WASP-depleted tumours survived significantly longer. | Pancreatic ductal adenocarcinoma99 | ||
| RAC1 | Regulates macropinocytosis, membrane trafficking and cellular morphology | RAC1 activation leads to cancer cell proliferation/survival, actin remodelling/migration (EMT transition phenotype), metastasis in vivo and angiogenesis. | Breast cancer,100,101 gastric adenocarcinoma102 |
| CDC42 | Involved in intracellular trafficking, ER–Golgi interface trafficking (both anterograde and retrograde), post-Golgi transport and exocytosis | Activation/overexpression promoted tumour progression and metastasis in different tumour types in vivo. | NSCLC, gastric cancer, breast cancer103 |
| RAB subfamily: | |||
| RAB1 (RAB1A and RAB1B) | Regulates ER-Golgi traffic | Loss of RAB1B expression in triple-negative breast cancer correlates with higher metastasis. | Breast cancer,104 NSCLC, gastric cancer, and oesophageal squamous cell carcinoma105 |
| RAB2 | Retrograde transfer of vesicles | RAB2A overexpression causes increased cellular invasiveness and acquisition of EMT traits. | Breast cancer106 |
| RAB3 | Modulates secretion of vesicles leading to exocytosis | RAB3C overexpression promotes migration, invasion and metastasis in vivo. | Colorectal cancer107 |
| RAB3D promotes breast cancer cell invasion and lung metastasis in vivo through activation of the AKT/GSK-3β/Snail signalling pathway. | Breast cancer108 | ||
| RAB4 | Regulates recycling of vesicles | RAB4 recycling route is central in promoting invasive properties of cancer cells through integrin β3. | Breast cancer109 |
| RAB5, RAB21 and RAB22 | Regulates early endosome trafficking | RAB5 promotes integrin trafficking, focal adhesion turnover, RAC1 activation, tumour cell migration and invasion. | Breast cancer,110 colon adenocarcinoma and melanoma111 |
| RAB5 mediates hypoxia-driven tumour cell migration, invasion and metastasis in vivo. | Breast cancer and melanoma112 | ||
| RAB21 regulates integrin-mediated cell adhesion and motility. | Cervical cancer cells113 | ||
| RAB22 and RAB163 (C-terminal of BRCA2 protein) specifically interact with the RAD51 protein. | Affects breast cancer susceptibility gene (BRCA2)114 | ||
| TBC1D2b (a RAB22 GTPase-activating protein) is suppressed by ZEB1/NuRD complex to increase E-cadherin internalisation and promote metastasis in lung cancer using subcutaneous syngeneic mice model. | NSCLC115 | ||
| RAB6 | Regulates anterograde and retrograde trafficking routes between the Golgi apparatus, endoplasmic reticulum, plasma membrane, and endosomes | Elevated expression of RAB5A correlates with either poor or favourable prognosis. | Colorectal cancer,116 gastric cancer117 |
| Knockdown promotes cell migration by inhibiting myosin II phosphorylation and upregulating Cdc42 activity. | Bone osteosarcoma, NSCLC cells118 | ||
| RAB7 | Regulates late endocytic pathway, including endosome maturation, early endosomes to late endosomes transition, clustering and fusion to lysosomes | RAB7 downregulation is important for acquisition of invasive properties in melanoma cells and correlates with increased risk of metastasis development. | Melanoma119 |
| RAB11 | Regulates membrane protein recycling and protein transport from TGN to the plasma membrane (slow recycling) | Regulates RAC activity and polarisation during collective cell migration, hypoxia-stimulated cell invasion in cancer cells. | Breast cancer120 |
| RCP (a RAB11 effector)-dependent trafficking of Eph receptor drives cell–cell repulsion and metastasis in an autochthonous mouse model of pancreatic adenocarcinoma. | Pancreatic adenocarcinoma121 | ||
| RAB27 | Regulates secretory pathway/exocytosis and melanosomes | Both the isoforms RAB27A and RAB27B, are known to promote cell invasion and tumour metastasis in vivo. | Bladder cancer, melanoma and breast cancer cells122 |
| RAB35 | Regulates fast recycling of proteins to the plasma membrane and in sorting endosomes | Constitutively active RAB35 is proposed to be oncogenic due to activation of PI3K/Akt signalling. Regulates cancer cell migration and invasion. | Gastric cancer, cervical cancer cells123 |
| Mutant p53 drives metastasis in autochthonous mouse models of pancreatic cancer by controlling the production of sialomucin, podocalyxin and activity of the RAB35 GTPase, which interacts with podocalyxin to influence its sorting to exosomes. These exosomes influence integrin trafficking in normal fibroblasts to promote deposition of a highly pro-invasive ECM. | Pancreatic cancer and NSCLC124 | ||
ECM extracellular matrix, PI3K phosphoinositide 3-kinase, RCP RAB-coupling protein, TGN trans-Golgi network, GSK-3β glycogen synthase kinase 3β, EMT epithelial-to-mesenchymal transition, CXCR-2 C-X-C chemokine receptor-2, NSCLC non-small cell lung cancer, FAK focal adhesion kinase, CCR5 CC- chemokine receptor 5.
Clathrin-mediated endocytosis (CME)
The most studied endocytic mechanism is CME. It was first found to play an important role in low-density lipoprotein6 and transferrin uptake.7 It is known to be involved in internalisation and recycling of multiple receptors engaged in signal transduction (G-protein and tyrosine-kinase receptors), nutrient uptake and synaptic vesicle reformation.8 Clathrin-coated pits (CCP) are assemblies of cytosolic coat proteins, which are initiated by AP2 (assembly polypeptide 2) complexes that are recruited to a plasma membrane region enriched in phosphatidylinositol-(4,5)-bisphosphate lipid.9 AP2 acts as a principal cargo-recognition molecule and recognises internalised receptors through a short sequence motif in their cytoplasmic domains.10 As the nascent invagination grows, AP2 and other cargo-specific adaptor proteins recruit and concentrate the cargo, which is now facing the inside of the vesicle. Following cargo recognition/concentration, AP2 complexes along with other adaptor proteins to recruit clathrin. Clathrin recruitment stabilises the curvature of the growing CCP with the help of BAR (Bin-Amphiphysin-Rvs)-domain-containing proteins until the entire region invaginates to form a closed vesicle.11
Release of mature clathrin-coated vesicles from the plasma membrane is performed by the large multi-domain GTPase, Dynamin. Proteins such as amphiphysin, endophilin and sorting nexin 9 (BAR-domain-containing proteins) recruit Dynamin around the necks of budding vesicles.12 Similarly, other Dynamin partners (i.e., Grb2) also bind to Dynamin and increase its oligomerisation, which results in a higher GTPase activity.13 Oligomerised Dynamin assembles into collar-like structures encircling the necks of deeply invaginated pits and undergoes GTP hydrolysis to drive membrane fission.14 After a vesicle is detached from the plasma membrane, the clathrin coat is disassembled by the combined action of the ATPase HSC70 and the coat component auxilin.15,16 The released uncoated vesicle is ready to travel and fuse with its target endosome.
Signalling through CME is critical in cancer and metastasis. Clathrin light-chain isoform (CLCb) is specifically upregulated in non-small-cell lung cancer (NSCLC) cells and is associated with poor prognosis. NSCLC cells expressing CLCb exhibit increased rates of CME through Dynamin 1. This leads to activation of a positive feedback loop involving enhanced epidermal growth factor receptor (EGFR)-dependent Akt/GSK-3β (glycogen synthase kinase 3β) phosphorylation, resulting in increased cell migration and metastasis.17 Dynamin 2 is crucial for the endocytosis of several proteins known to be involved in cancer motility and invasiveness (e.g., β-1 integrin and focal adhesion kinase). Dynamin 2 overexpression correlates with poor prognosis.18
The regulation of certain receptors that are known to affect cancer and metastasis (i.e., EGFR and transforming growth factor β receptor (TGFβR)) by clathrin- and non-clathrin-mediated internalisation pathways preferentially targets the receptors to different fates (i.e., recycling or degradation).19,20 Different fates of receptors determine the net signalling output in a cell and affect cancer progression. Interestingly, CME is known to skew EGFR fate towards recycling rather than degradation, leading to prolonged duration of signalling.20 Similarly, the internalised EGF–EGFR complex may maintain its ability to generate cell signalling from endosomes affecting multiple downstream pathways.21 This active endosomal EGFR is known to regulate oncogenic Ras activity by co-internalising its regulators including Grb2, SHC, GAP and Cbl.21,22
Caveolae-mediated endocytosis (CavME)
CavME is the second most studied pathway of endocytosis and has been shown to be important in transcytotic trafficking across cells and mechanosensing.23 The CavME process involves formation of a bulb-shaped, 50–60-nm plasma membrane invaginations called caveolae (little caves), which is driven by both integral membrane proteins called caveolins and peripheral membrane proteins called cavins (cytosolic coat proteins). Caveolins (encoded by CAV-1, 2 and 3 paralogues) are small integral membrane proteins that are inserted into the inner side of the membrane bilayer through its cytosolic N-terminal region that binds to cholesterol. About 50 cavin molecules associate with each caveolae and exist in a homo- or hetero-oligomeric form (using four cavin family members).24 CavME is triggered by ligand binding to cargo receptors concentrated in caveolae. Budding of caveolae from the plasma membrane is regulated by kinases and phosphatases, such as Src tyrosine kinases and serine/threonine protein phosphatases PP1 and PP2A.25 As with CME, Dynamin is required to pinch off caveolae vesicles from the plasma membrane.26
Components of CavME have a vital role in cell migration, invasion and metastasis. It is speculated that CAV-1 has a dual role in cancer progression and metastasis. In the early stages of the disease, it functions predominantly as a tumour suppressor, whereas at later stages, its expression is associated with tumour progression and metastasis.27–29 As with a tumour suppressor, CAV-1 is often deleted in human cancers and mechanistically known to act through the caveolin scaffolding domain (CSD) by inhibiting cytokine receptor signalling.28,30 The CAV-1 effect on the late-stage tumour progression and metastasis has been attributed to tyrosine (Tyr14) phosphorylation of its protein product by Src kinases, leading to increased Rho/ROCK signalling and subsequent focal adhesion turnover.31 Knockdown of CAV-1 in breast and prostate cancer cells reduced the velocity, directionality and persistency of cellular migration.31,32 Similarly, expression of CAV-1 has been used as a marker of prognosis and overall survival in various types of human cancer. In pancreatic adenocarcinoma, positive expression of CAV-1 was found to correlate with tumour diameter, histopathological grade and poor prognosis. In lung cancer, CAV-1 expression statistically correlates with poor differentiation, pathological stage, lymph-node metastasis and poor prognosis. However, in hepatocellular carcinoma tissues, low expression of CAV-1 is associated with poor prognosis.33
Clathrin-independent endocytosis (CIE)
As per the name, the endocytic vesicles involved in CIE have no distinct coat and were first discovered by their resistance to inhibitors that block CME and CavME.34 CIE encompasses several pathways. (i) An endophilin-, Dynamin- and RhoA-dependent pathway for endocytosis of interleukin-2 receptor.35 (ii) A clathrin- and Dynamin-independent (CLIC/GEEC) pathway in which the GTPases RAC1 and CDC42 lead to actin-dependent formation of clathrin-independent carriers (CLICs). This, in turn, forms the glycosylphosphatidylinositol (GPI)-AP-enriched endosomal compartments (GEECs).36,37 (iii) An ARF6-dependent pathway involving the small GTPase ARF6, to activate phosphatidylinositol-4-phosphate 5-kinase that produces phosphatidylinositol-(4,5)-bisphosphate, leading to stimulation of actin assembly and endocytosis.38 The CIE pathway has been shown to suppress cancer cell blebbing and invasion through GTPase-activating protein GRAF1 (GTPase regulator associated with focal adhesion kinase-1) (Table 1).39 Various receptors are endocytosed by the CIE pathway, including interleukin-2 receptor (IL-2R), T-cell receptor (TCR) and GPI-linked proteins.40
Downstream endosomal trafficking
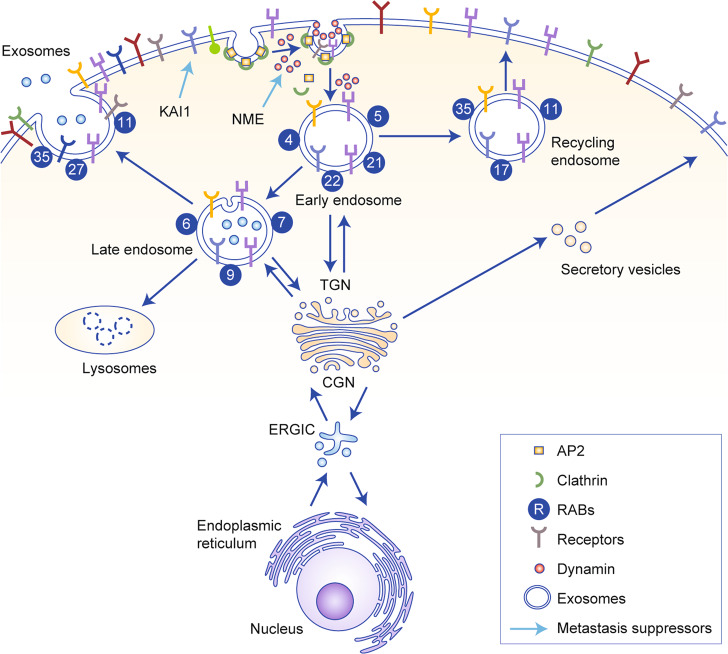
Internalised receptor–ligand cargoes can merge into a common endosomal network by undergoing multiple rounds of fusions. The first set of fusion leads to the formation of early endosomes where initial sorting routes are engaged, and the fate of the internalised receptors is decided (Fig. 1). Early endosomes are identified by the association of several proteins on their cytosolic surface, including RAB5, along with its effector VPS34/p150, a phosphatidylinositol 3-kinase complex. VPS34/p150 generates phosphatidylinositol 3-phosphate, which regulates the spatiotemporal and compartmentalisation aspects of endosomal functions.41,42 Structurally, early endosomes have tubular (membrane) and vacuolar (vacuoles) domains. Most of the membrane surface area lies in the tubules, while much of the volume is in the vacuoles. The membrane domains are enriched in proteins, including RAB5, RAB4, RAB11, ARF1/COPI, retromer and caveolin.43,44 These proteins are involved in multiple functions, including molecular sorting of early endosomes to distinct organelles, its recycling and maturation to late endosomes or to the trans-Golgi network (TGN) (Fig. 1). The role of these endocytic proteins in metastasis in vivo and their prognostic potential, if any, have been listed in Table 1.
Fig. 1. Endosomal trafficking and metastasis suppressor genes.
A wide variety of receptors and their ligands are moved intracellularly by endocytosis. Clathrin-mediated endocytosis begins with initiation and maturation of clathrin-coated pits by AP2 complexes that are recruited to the plasma membrane and act as a principal cargo-recognition molecule. As the nascent invagination grows, AP2 and other cargo-specific adaptor proteins recruit and concentrate the cargo. AP2 complexes along with other adaptor proteins to recruit clathrin. Clathrin recruitment stabilises the curvature of the growing pit with the help of other BAR-domain-containing proteins. BAR-domain-containing proteins also recruit Dynamin to the neck of the budding vesicle, until the entire region invaginates to form a closed vesicle. Dynamin is a large GTPase, which forms a helical oligomer around the constricted neck and, upon GTP hydrolysis, mediates the fission of the vesicle to release it into the cytoplasm. Following vesicle detachment from the plasma membrane, the clathrin coat is disassembled. The released vesicle goes through a first set of fusion, leading to formation of early endosomes, where initial sorting decisions are made, and the fate of the internalised sorting proteins and lipids is decided. The RAB proteins primarily localised to the early endosome include RAB5 and RAB4, along with lesser-known RAB21 and RAB22. They regulate the motility of early endosome on actin and microtubule tracks, its homotypic fusion and specialised functions of sorting and trafficking. The internalised receptors can be sorted into recycling pathways through extensive tubulation of the early endosome membranes, wherein receptors that are sorted into the newly formed tubular membranes recycle back to the plasma membrane through recycling endosomes. Alternately, early endosome growth and maturation could lead to the trans-Golgi network (TGN) or to late endosomes. Mature late endosomes are approximately 250–1000 nm in diameter and are characterised by the generation of a RAB7 domain. Late endosomes undergo homotypic fusion reactions, grow in size and acquire more intralumenal vesicles (ILVs). ILVs containing late endosomes get enriched with RAB35 and RAB27 and their effectors that promote their fusion to plasma membrane to release exosomes (40–100 nm in diameter vesicles). Predominantly, late endosomes move to the perinuclear area of the cell where they undergo transient fusions with each other and eventually fuse with lysosomes for degradation of its content. Cellular proteins synthesised in the rough endoplasmic reticulum (ER) are constantly secreted from ER to the Golgi complex in mammals through an ER–Golgi intermediate compartment (ERGIC). Points where metastasis suppressors interact with the endocytic process are highlighted.
A recycling pathway returns endosomes to the cell surface either by a fast recycling route (via RAB4-positive endosomes) or by a slow recycling route (via RAB11-positive endosomes).45 Internalised receptors in early endosomes can be sorted into the recycling pathway through an extensive tubulation of the early endosome membranes in a process called ‘geometry-based sorting’ wherein receptors that are sorted into the newly formed tubular membranes of the early endosome are recycled back to the plasma membrane. Intralumenal vesicles (ILVs) also form in early endosomes, driven by clathrin and components of the endosomal sorting complex required for transport (ESCRT).46 ESCRT-mediated receptor sorting into ILVs is an evolutionarily conserved process that is required for multivesicular body (MVB) formation. ESCRT uses its various complexes for receptor recognition (ESCRT-0), inward budding (ESCRT-I and II) and final ESCRT-III-mediated abscission.47 This separates the cytoplasmic portion of the receptors from the rest of the cell, leading to abrogation of its signalling. Interestingly, depletion of ESCRT-0 and ESCRT-I subunits inhibits the degradation of EGFR and results in enhanced recycling and sustained activation of extracellular signal-regulated kinase (ERK) signalling.48,49
A role for endosomal acidification and ligand dissociation has also been established. Recycling of receptors to the plasma membrane takes place if the ligands are released in the early endosome (i.e., transferrin receptor), where the pH is maintained at ~6.5.50 Conversely, some signalling receptors (i.e., EGFR) often retain ligand binding and remain active even at low (~4.5) pH, leading to their continual signalling from endosomal compartments until they are sorted into ILVs and degraded in the lysosome.51
Some internalised receptors in early endosomes can be sorted to the TGN in a process called retrograde transport (i.e., mannose-6-phosphate receptors and several toxins such as Shiga, cholera and ricin). The TGN is a network of interconnected tubules and vesicles at the trans-face of the Golgi apparatus. It is essential for maintaining cellular homoeostasis and is known to play a crucial role in protein sorting or diverting proteins and lipids away from lysosomal degradation.
Mature late endosomes are approximately 250–1000 nm in diameter and are round/oval in shape. They are characterised by the presence of RAB7-GTPase, which is fundamental for the maturation of early-to-late endosomes and for the lysosomal biogenesis. Maturation of early-to-late endosomes depends on the formation of a hybrid RAB5/RAB7 endosome, wherein RAB7 is recruited to the early endosome by RAB5-GTP.52 Late endosomes undergo homotypic fusion reactions, grow in size and acquire more intraluminal vesicles. Once intralumenal vesicles containing late endosomes become enriched with RAB35, RAB27A, RAB27B and their effectors Slp4 and Slac2b, they fuse to plasma membrane to release exosomes.37 The released exosomes are small (40–100 nm in diameter), single membrane-bound vesicles that contain protein, DNA and RNA. Mostly, however, late endosomes move to the perinuclear area of the cell in the vicinity of lysosomes using dynein-dependent transport. Here, late endosomes undergo transient fusions with each other and eventually fuse with lysosomes to generate a transient hybrid organelle called the endolysosome. It is in the endolysosomes in which most of the hydrolysis of endocytosed cargo takes place.37 Following a further maturation process, the endolysosome is converted into a classical dense lysosome.
Cellular contents and organelles can also be delivered to lysosomes through a separate pathway called autophagy. Autophagy or self-eating is a unique membrane trafficking process whereby a newly formed isolation membrane can elongate and engulf part of the cytoplasm or organelles to form autophagosomes that are delivered to the lysosome for degradation. There are an increasing number of reports pointing to a mechanistic role for autophagy in the process of tumour metastasis, detailed in a recent review.53
An astonishing number of endosomal trafficking pathway proteins are known to be functionally important in tumour progression and metastasis (Table 1). Many have been validated in cancer cell motility and invasion, but a considerable number have been shown to modulate in vivo metastasis. The alterations identified include up- or down-regulation of expression, or mutation, and generally lead to an aberrant receptor trafficking/recycling/degradation/signal duration, which has a profound effect on cancer cell migration, invasion and/or proliferation. While most of these reports focus on a single signalling pathway, it is likely that multiple pathways are also affected. These mechanistic studies cover a wide range of cancer types. Additional details on different endosomal trafficking members and their role(s) in cancer and metastasis can be found in recent reviews.54–56
Integrin and extracellular matrix trafficking in metastasis
Cancer cells invade through the extracellular matrix (ECM) in part by producing matrix metalloproteinases (MMPs) and other proteinases that degrade the ECM, thereby creating paths for migration. Similarly, cells attach to the ECM by means of integrins that are key regulators of cell adhesion, migration and proliferation. The interplay between integrins and ECM remodelling proteases is a major regulator of tumour invasion.
In oral squamous cell carcinoma (SCC), increased αVβ6 integrin expression leads to the activation of MMP-3 and promotes oral SCC cell proliferation and metastasis in vivo.57 MMP-14 (membrane type 1 metalloprotease MT1-MMP), along with integrin αVβ3 co-localised to the protruding ends of invadopodia, and its high local concentration on the cell membrane promoted metastasis.58 Interestingly, WDFY2 (a cytosolic protein) controls the recycling of MT1-MMP to the membrane, and loss of WDFY2 leads to enhanced secretion of MT1-MMP leading to active invasion of cells.59
Recent studies highlight the importance of integrin trafficking (endocytosis and recycling) as a modulator of cancer cells’ fate. For example, rapid recycling of integrins from the leading edge of individual cells assists in efficient cell motility by providing a supply of fresh receptors that are internalised at the trailing edge. More details on the trafficking of MMPs and integrins and its role in metastasis can be found in recent reviews.60,61
Metastasis suppressors and endocytosis
Metastasis suppressors are a group of genes that suppress the metastatic potential of cancer cells without significantly affecting the size of primary tumour.62 So far, more than 20 metastasis suppressor genes (including miRNAs) have been identified in multiple cancer types with a wide range of biochemical activities.63 Some of the metastasis suppressor genes working through alterations in endocytosis are described below:
NME1 (NM23/NM23-H1, non-metastatic clone 23, isoform H1)
NME is a multifunctional protein that is highly conserved from yeast to humans. Its enforced expression suppressed metastasis in a variety of cancer cell lines without altering primary tumour growth.64 Apart from being a metastasis suppressor, it is also known to have a developmental function.
The Drosophila homologue of NME is awd (abnormal wing discs) and is known to regulate cell differentiation and motility of multiple organs in late embryogenesis by regulating growth factor receptor signalling through endocytosis. These studies identified a genetic interaction between awd and dynamin (shi).65 An aberrant endocytosis was associated with mutant awd phenotypes and complemented RAB5 or shi genes.65–67 It was also shown that awd regulated tracheal cell motility in development by modulating the fibroblast growth factor receptor (FGFR) levels through dynamin-mediated endocytosis.65,68 Interestingly, loss of awd gene also blocked Notch signalling by altering the receptor processing that leads to Notch accumulation in the early endosomes.67
Recent reports in mammalian cancer models have also highlighted the role of NME as an interacting partner of Dynamin in endocytosis.69,70 NME transfectants of multiple cell lines exhibited increased endocytosis of EGFR and transferrin in concert with motility suppression. Both the increased endocytic and motility-suppression phenotypes were blocked by inhibitors of Dynamin. In a lung-metastasis assay, NME1 overexpression failed to significantly suppress metastasis in cells in which Dynamin 2 was also knocked down. Using the EGF/EGFR signalling axis as an in vitro model, NME1 decreased the phospho-EGFR and phospho-Akt levels in a Dynamin 2-dependent manner, highlighting the relevance of this interaction for downstream signalling. It was speculated that NME acted as a GTP provider/oligomerising agent of Dynamin 2, leading to higher Dynamin 2 GTPase activity and increased endocytosis (Fig. 1).69,70 Our data identified another function of a NME–Dynamin interaction: in vitro, NME promoted the oligomerisation of Dynamin and its increased GTPase activity, which are needed for vesicle scission.69
KAI1 (CD82, cluster of differentiation 82)
KAI1/CD82 is a member of the evolutionarily conserved tetraspanin family, and was initially identified as a metastasis suppressor in prostate cancer.71 KAI1 has since been established as a metastasis suppressor in a variety of solid tumours. Its higher expression predicts a better prognosis,72–74 whereas reduced expression of KAI1 has been widely correlated with an aggressive cancer in several cancer types, including pancreatic, hepatocellular, bladder, breast and non-small-cell lung cancers.73,75,76
KAI1-mediated suppression of metastasis is thought to be achieved primarily by inhibiting cancer cell migration and invasion.77 This phenotype is the result of forming oligomeric complexes with binding partners such as integrins, EGFR and intracellular signalling proteins, such as protein kinase C (PKC). This complex generally leads to either redistribution or increased internalisation of multiple receptors. For example, overexpression of KAI1 leads to redistribution of urokinase-type plasminogen activator receptor (uPAR) into a stable complex with integrin α5β1 in focal adhesions.78 Focal adhesion binding of uPAR reduces its ability to bind the ligand uPA and consequently to cleave and activate plasminogen. Similarly, KAI1 also binds with EGFR, ErbB2 and ErbB3; for EGFR, this leads to accelerated endocytosis and desensitisation.79,80 KAI1 also specifically inhibits ligand-induced EGFR dimerisation and alters the distribution of EGFR in the plasma membrane, which consequently affects its activation.80
MTSS1 (metastasis suppressor protein 1 or MIM, missing in metastasis)
MTSS1/MIM, originally identified in bladder cancer cell lines, was present in non-metastatic but not metastatic bladder cancer cells.81 It is hypothesised that MTSS1 suppresses metastasis by acting as a scaffold protein to interact with actin-associated proteins to regulate cytoskeletal dynamics and lamellipodia formation, consequently affecting invasion and metastatic behaviour of cancer cells.82 In head and neck squamous cell carcinoma, MTSS1 augments EGF signalling by antagonising EGFR endocytosis at low cell densities and promotes cellular proliferation at early stages of primary head and neck squamous cell carcinoma tumour growth. However, at high cell densities, MTSS1 has a negative impact on EGF signalling and inhibits metastasis.83
KISS1 (kisspeptin-1)
The KISS1 gene produces a peptide product called kisspeptins (KP), which act as an endogenous ligand for a G-protein-coupled receptor, KISS1R (GPR54).84 KISS1 acts as a metastasis suppressor gene through its KP/KISS1R signalling in numerous human cancers (melanoma, pancreatic cancer and gastric carcinoma) by inhibiting cellular motility, proliferation, invasion, chemotaxis and metastasis.85 However, in breast cancer, KP stimulates invasion of cancer cells and high expression of KISS1; GPR54 mRNA levels positively correlated with shorter relapse-free survival. Interestingly, GPR54 directly complexes with EGFR, and stimulation of breast cancer cells by either EGF or KP-10 regulated the endocytosis of both GPR54 and EGFR.86 This signalling has an opposite effect on breast cancer cells, i.e., it is pro-migratory and pro-invasive in human breast cancer cells.
Metastasis suppressor genes, while often showing statistically significant inverse trends of tumour expression and patient survival, are not likely to become clinically used prognostic factors, in favour of more complex gene signatures. As with tumour suppressors, their translation to the clinic has also been problematic. Restoration of metastasis suppressor expression in every metastatic tumour cell would be needed for optimal activity, which is unrealistic. Our laboratory explored the transcriptional upregulation of NME by high-dose medroxyprogesterone acetate.87 A Phase 2 trial, conducted at Indiana University, was a technical failure, as serum levels of medroxyprogesterone acetate were not sufficiently elevated, although some long-term stable disease was observed.88 How the endocytic pathways can contribute to a metastatic-suppressor clinical–translational effort is currently unknown but of high interest. More research to identify the complex mechanisms underlying these processes is warranted.
Conclusions
Endocytosis is a process of internalisation of the plasma membrane along with its membrane proteins and lipids. Cells use endocytosis to regulate signalling and to sample the extracellular milieu for appropriate responses. It affects almost all of the steps of metastasis and is used as a tool for the functioning of metastasis suppressors. Based on the literature, endocytosis regulates receptor internalisation, recycling and degradation, or could affect cytoskeleton dynamics to alter cancer cell invasion or metastasis. However, the majority of the above conclusions have been made based on studies conducted on cancer cell lines. These studies would benefit from validation on patient-derived tissues. Other challenges in this field are a lack of high-resolution knowledge of the endosomal sorting complexes and their central regulators, and how signalling in cancer cells is altered at specific stages of endocytosis. These issues will undoubtedly be clarified as research progresses. Identification of these central regulators could serve as trafficking nodes that are amenable to therapeutic interception. A potential issue in translation is the effect of an inhibitor of an endocytic node on multiple signalling pathways that it engages, and how the cumulative effects modulate the metastatic phenotype. This issue is not unique to endocytosis and applies to DNA methylation and other cancer processes. In summary, targeting the endocytic machinery could be a viable and promising therapeutic strategy for cancer and metastasis.
Author contributions
I.K. and P.S.S. reviewed the literature, drafted and revised the paper.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Funding information
This work is supported by the NIH Intramural program.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal, A., Ward, E. M., Johnson, C. J., Cronin, K. A., Ma, J., Ryerson, B. et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J. Natl Cancer Inst.109, djx030 (2017). [DOI] [PMC free article] [PubMed]
- 3.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 4.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev. Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 5.Haigler HT, McKanna JA, Cohen S. Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J. Cell Biol. 1979;83:82–90. doi: 10.1083/jcb.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpentier JL, Gorden P, Anderson RG, Goldstein JL, Brown MS, Cohen S, et al. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J. Cell Biol. 1982;95:73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neutra MR, Ciechanover A, Owen LS, Lodish HF. Intracellular transport of transferrin- and asialoorosomucoid-colloidal gold conjugates to lysosomes after receptor-mediated endocytosis. J. Histochem Cytochem. 1985;33:1134–1144. doi: 10.1177/33.11.2997327. [DOI] [PubMed] [Google Scholar]
- 8.Takei K, Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 9.Traub LM, Bonifacino JS. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 2013;5:a016790. doi: 10.1101/cshperspect.a016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr. Opin. Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Qualmann B, Koch D, Kessels MM. Let’s go bananas: revisiting the endocytic BAR code. EMBO J. 2011;30:3501–3515. doi: 10.1038/emboj.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barylko B, Binns D, Lin KM, Atkinson MA, Jameson DM, Yin HL, et al. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J. Biol. Chem. 1998;273:3791–3797. doi: 10.1074/jbc.273.6.3791. [DOI] [PubMed] [Google Scholar]
- 14.Schmid SL, Frolov VA. Dynamin: functional design of a membrane fission catalyst. Annu Rev. Cell Dev. Biol. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- 15.Newmyer SL, Schmid SL. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J. Cell Biol. 2001;152:607–620. doi: 10.1083/jcb.152.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- 17.Chen PH, Bendris N, Hsiao YJ, Reis CR, Mettlen M, Chen HY, et al. Crosstalk between CLCb/Dyn1-mediated adaptive clathrin-mediated endocytosis and epidermal growth factor receptor signaling increases metastasis. Dev. Cell. 2017;40:278–288 e275. doi: 10.1016/j.devcel.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B, Teng LH, Silva SD, Bijian K, Al Bashir S, Jie S, et al. The significance of dynamin 2 expression for prostate cancer progression, prognostication, and therapeutic targeting. Cancer Med. 2014;3:14–24. doi: 10.1002/cam4.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 20.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol. Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parton RG, Simons K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 24.Gambin Y, Ariotti N, McMahon KA, Bastiani M, Sierecki E, Kovtun O, et al. Single-molecule analysis reveals self assembly and nanoscale segregation of two distinct cavin subcomplexes on caveolae. Elife. 2013;3:e01434. doi: 10.7554/eLife.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiss AL. Caveolae and the regulation of endocytosis. Adv. Exp. Med Biol. 2012;729:14–28. doi: 10.1007/978-1-4614-1222-9_2. [DOI] [PubMed] [Google Scholar]
- 26.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quest AF, Gutierrez-Pajares JL, Torres VA. Caveolin-1: an ambiguous partner in cell signalling and cancer. J. Cell Mol. Med. 2008;12:1130–1150. doi: 10.1111/j.1582-4934.2008.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 29.Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- 30.Engelman JA, Zhang XL, Galbiati F, Lisanti MP. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31) FEBS Lett. 1998;429:330–336. doi: 10.1016/s0014-5793(98)00619-x. [DOI] [PubMed] [Google Scholar]
- 31.Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–8220. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 32.Urra H, Torres VA, Ortiz RJ, Lobos L, Diaz MI, Diaz N, et al. Caveolin-1-enhanced motility and focal adhesion turnover require tyrosine-14 but not accumulation to the rear in metastatic cancer cells. PLoS ONE. 2012;7:e33085. doi: 10.1371/journal.pone.0033085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D, Che G. Value of caveolin-1 in cancer progression and prognosis: emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (Review) Oncol. Lett. 2014;8:1409–1421. doi: 10.3892/ol.2014.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J. Cell Biol. 1985;101:548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell. 2001;7:661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 36.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011;12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holst MR, Vidal-Quadras M, Larsson E, Song J, Hubert M, Blomberg J, et al. Clathrin-independent endocytosis suppresses cancer cell blebbing and invasion. Cell Rep. 2017;20:1893–1905. doi: 10.1016/j.celrep.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Mayor, S., Parton, R. G. & Donaldson, J. G. Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol.6, a016758 (2014). [DOI] [PMC free article] [PubMed]
- 41.Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 42.Zerial M, McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 43.Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J. Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 2010;191:615–629. doi: 10.1083/jcb.201003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 46.Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 47.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 48.Malerod L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 49.Raiborg C, Malerod L, Pedersen NM, Stenmark H. Differential functions of Hrs and ESCRT proteins in endocytic membrane trafficking. Exp. Cell Res. 2008;314:801–813. doi: 10.1016/j.yexcr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs R, Male P, Mellman I. Acidification and ion permeabilities of highly purified rat liver endosomes. J. Biol. Chem. 1989;264:2212–2220. [PubMed] [Google Scholar]
- 51.Mellman I. Endocytosis and molecular sorting. Annu Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 52.Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 53.Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2017;36:1619–1630. doi: 10.1038/onc.2016.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mellman I, Yarden Y. Endocytosis and cancer. Cold Spring Harb. Perspect. Biol. 2013;5:a016949. doi: 10.1101/cshperspect.a016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanzetti L, Di Fiore PP. Endocytosis and cancer: an ‘insider’ network with dangerous liaisons. Traffic. 2008;9:2011–2021. doi: 10.1111/j.1600-0854.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 56.Schmid SL. Reciprocal regulation of signaling and endocytosis: Implications for the evolving cancer cell. J. Cell Biol. 2017;216:2623–2632. doi: 10.1083/jcb.201705017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Yang Y, Hu Y, Dang D, Regezi J, Schmidt BL, et al. Alphavbeta6-Fyn signaling promotes oral cancer progression. J. Biol. Chem. 2003;278:41646–41653. doi: 10.1074/jbc.M306274200. [DOI] [PubMed] [Google Scholar]
- 58.Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc. Natl Acad. Sci. USA. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sneeggen M, Pedersen NM, Campsteijn C, Haugsten EM, Stenmark H, Schink KO. WDFY2 restrains matrix metalloproteinase secretion and cell invasion by controlling VAMP3-dependent recycling. Nat. Commun. 2019;10:2850. doi: 10.1038/s41467-019-10794-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev. 2007;26:567–578. doi: 10.1007/s10555-007-9078-7. [DOI] [PubMed] [Google Scholar]
- 61.Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44-46:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan I, Steeg PS. Metastasis suppressors: functional pathways. Lab. Invest. 2018;98:198–210. doi: 10.1038/labinvest.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurst DR, Welch DR. Metastasis suppressor genes at the interface between the environment and tumor cell growth. Int Rev. Cell Mol. Biol. 2011;286:107–180. doi: 10.1016/B978-0-12-385859-7.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salerno M, Ouatas T, Palmieri D, Steeg PS. Inhibition of signal transduction by the nm23 metastasis suppressor: possible mechanisms. Clin. Exp. Metastasis. 2003;20:3–10. doi: 10.1023/a:1022578000022. [DOI] [PubMed] [Google Scholar]
- 65.Dammai V, Adryan B, Lavenburg KR, Hsu T. Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev. 2003;17:2812–2824. doi: 10.1101/gad.1096903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woolworth JA, Nallamothu G, Hsu T. The Drosophila metastasis suppressor gene Nm23 homolog, awd, regulates epithelial integrity during oogenesis. Mol. Cell Biol. 2009;29:4679–4690. doi: 10.1128/MCB.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ignesti M, Barraco M, Nallamothu G, Woolworth JA, Duchi S, Gargiulo G, et al. Notch signaling during development requires the function of awd, the Drosophila homolog of human metastasis suppressor gene Nm23. BMC Biol. 2014;12:12. doi: 10.1186/1741-7007-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nallamothu G, Woolworth JA, Dammai V, Hsu T. Awd, the homolog of metastasis suppressor gene Nm23, regulates Drosophila epithelial cell invasion. Mol. Cell Biol. 2008;28:1964–1973. doi: 10.1128/MCB.01743-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan I, Gril B, Steeg PS. Metastasis suppressors NME1 and NME2 promote dynamin 2 oligomerization and regulate tumor cell endocytosis, motility, and metastasis. Cancer Res. 2019;79:4689–4702. doi: 10.1158/0008-5472.CAN-19-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boissan M, Montagnac G, Shen Q, Griparic L, Guitton J, Romao M, et al. Membrane trafficking. Nucleoside diphosphate kinases fuel dynamin superfamily proteins with GTP for membrane remodeling. Science. 2014;344:1510–1515. doi: 10.1126/science.1253768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ichikawa T, Ichikawa Y, Isaacs JT. Genetic factors and suppression of metastatic ability of prostatic cancer. Cancer Res. 1991;51:3788–3792. [PubMed] [Google Scholar]
- 72.Dong JT, Isaacs WB, Isaacs JT. Molecular advances in prostate cancer. Curr. Opin. Oncol. 1997;9:101–107. doi: 10.1097/00001622-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, et al. Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 1996;56:4387–4390. [PubMed] [Google Scholar]
- 74.Ow K, Delprado W, Fisher R, Barrett J, Yu Y, Jackson P, et al. Relationship between expression of the KAI1 metastasis suppressor and other markers of advanced bladder cancer. J. Pathol. 2000;191:39–47. doi: 10.1002/(SICI)1096-9896(200005)191:1<39::AID-PATH580>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 75.Kawana Y, Komiya A, Ueda T, Nihei N, Kuramochi H, Suzuki H, et al. Location of KAI1 on the short arm of human chromosome 11 and frequency of allelic loss in advanced human prostate cancer. Prostate. 1997;32:205–213. doi: 10.1002/(sici)1097-0045(19970801)32:3<205::aid-pros7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 76.Uchida S, Shimada Y, Watanabe G, Li ZG, Hong T, Miyake M, et al. Motility-related protein (MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph node metastasis in oesophageal squamous cell carcinoma. Br. J. Cancer. 1999;79:1168–1173. doi: 10.1038/sj.bjc.6690186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res. 2001;61:5284–5288. [PubMed] [Google Scholar]
- 78.Bass R, Werner F, Odintsova E, Sugiura T, Berditchevski F, Ellis V. Regulation of urokinase receptor proteolytic function by the tetraspanin CD82. J. Biol. Chem. 2005;280:14811–14818. doi: 10.1074/jbc.M414189200. [DOI] [PubMed] [Google Scholar]
- 79.Odintsova E, Sugiura T, Berditchevski F. Attenuation of EGF receptor signaling by a metastasis suppressor, the tetraspanin CD82/KAI-1. Curr. Biol. 2000;10:1009–1012. doi: 10.1016/s0960-9822(00)00652-7. [DOI] [PubMed] [Google Scholar]
- 80.Odintsova E, Voortman J, Gilbert E, Berditchevski F. Tetraspanin CD82 regulates compartmentalisation and ligand-induced dimerization of EGFR. J. Cell Sci. 2003;116:4557–4566. doi: 10.1242/jcs.00793. [DOI] [PubMed] [Google Scholar]
- 81.Lee YG, Macoska JA, Korenchuk S, Pienta KJ. MIM, a potential metastasis suppressor gene in bladder cancer. Neoplasia. 2002;4:291–294. doi: 10.1038/sj.neo.7900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woodings JA, Sharp SJ, Machesky LM. MIM-B, a putative metastasis suppressor protein, binds to actin and to protein tyrosine phosphatase delta. Biochem J. 2003;371:463–471. doi: 10.1042/BJ20021962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dawson JC, Timpson P, Kalna G, Machesky LM. Mtss1 regulates epidermal growth factor signaling in head and neck squamous carcinoma cells. Oncogene. 2012;31:1781–1793. doi: 10.1038/onc.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cvetkovic D, Babwah AV, Bhattacharya M. Kisspeptin/KISS1R system in breast cancer. J. Cancer. 2013;4:653–661. doi: 10.7150/jca.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beck BH, Welch DR. The KISS1 metastasis suppressor: a good night kiss for disseminated cancer cells. Eur. J. Cancer. 2010;46:1283–1289. doi: 10.1016/j.ejca.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zajac M, Law J, Cvetkovic DD, Pampillo M, McColl L, Pape C, et al. GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness. PLoS ONE. 2011;6:e21599. doi: 10.1371/journal.pone.0021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palmieri D, Halverson D, Ouatas T, Horak C, Salerno M, Johnson J, et al. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J. Natl Cancer Inst. 2005;97:632–642. doi: 10.1093/jnci/dji111. [DOI] [PubMed] [Google Scholar]
- 88.Miller KD, Althouse SK, Nabell L, Rugo H, Carey L, Kimmick G, et al. A phase II study of medroxyprogesterone acetate in patients with hormone receptor negative metastatic breast cancer: translational breast cancer research consortium trial 007. Breast Cancer Res. Treat. 2014;148:99–106. doi: 10.1007/s10549-014-3131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Azarnia Tehran, D., Lopez-Hernandez, T. & Maritzen, T. Endocytic adaptor proteins in health and disease: lessons from model organisms and human mutations. Cells8, 1345 (2019). [DOI] [PMC free article] [PubMed]
- 90.Reis CR, Chen PH, Srinivasan S, Aguet F, Mettlen M, Schmid SL. Crosstalk between Akt/GSK3beta signaling and dynamin-1 regulates clathrin-mediated endocytosis. EMBO J. 2015;34:2132–2146. doi: 10.15252/embj.201591518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boulay PL, Schlienger S, Lewis-Saravalli S, Vitale N, Ferbeyre G, Claing A. ARF1 controls proliferation of breast cancer cells by regulating the retinoblastoma protein. Oncogene. 2011;30:3846–3861. doi: 10.1038/onc.2011.100. [DOI] [PubMed] [Google Scholar]
- 92.Schlienger S, Ramirez RA, Claing A. ARF1 regulates adhesion of MDA-MB-231 invasive breast cancer cells through formation of focal adhesions. Cell Signal. 2015;27:403–415. doi: 10.1016/j.cellsig.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 93.Rainero E, Howe JD, Caswell PT, Jamieson NB, Anderson K, Critchley DR, et al. Ligand-occupied Integrin Internalization Links Nutrient Signaling to Invasive Migration. Cell Rep. 2015;10:398–413. doi: 10.1016/j.celrep.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 94.Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 2002;4:929–936. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- 95.Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 2001;20:4973–4986. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schlienger S, Campbell S, Pasquin S, Gaboury L, Claing A. ADP-ribosylation factor 1 expression regulates epithelial-mesenchymal transition and predicts poor clinical outcome in triple-negative breast cancer. Oncotarget. 2016;7:15811–15827. doi: 10.18632/oncotarget.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miao B, Skidan I, Yang J, You Z, Fu X, Famulok M, et al. Inhibition of cell migration by PITENINs: the role of ARF6. Oncogene. 2012;31:4317–4332. doi: 10.1038/onc.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalpana G, Figy C, Yeung M, Yeung KC. Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci. Rep. 2019;9:16351. doi: 10.1038/s41598-019-52746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Juin A, Spence HJ, Martin KJ, McGhee E, Neilson M, Cutiongco MFA, et al. N-WASP control of LPAR1 trafficking establishes response to self-generated LPA gradients to promote pancreatic cancer cell metastasis. Dev. Cell. 2019;51:431–445 e437. doi: 10.1016/j.devcel.2019.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 101.Morrison Joly M, Williams MM, Hicks DJ, Jones B, Sanchez V, Young CD, et al. Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis. Breast Cancer Res. 2017;19:74. doi: 10.1186/s13058-017-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoon C, Cho SJ, Chang KK, Park DJ, Ryeom SW, Yoon SS. Role of Rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol. Cancer Res. 2017;15:1106–1116. doi: 10.1158/1541-7786.MCR-17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Xiao, X. H., Lv, L. C., Duan, J., Wu, Y. M., He, S. J., Hu, Z. Z. et al. Regulating Cdc42 and Its signaling pathways in cancer: small molecules and microRNA as new treatment candidates. Molecules23, 787 (2018). [DOI] [PMC free article] [PubMed]
- 104.Jiang HL, Sun HF, Gao SP, Li LD, Hu X, Wu J, et al. Loss of RAB1B promotes triple-negative breast cancer metastasis by activating TGF-beta/SMAD signaling. Oncotarget. 2015;6:16352–16365. doi: 10.18632/oncotarget.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang XZ, Li XX, Zhang YJ, Rodriguez-Rodriguez L, Xiang MQ, Wang HY, et al. Rab1 in cell signaling, cancer and other diseases. Oncogene. 2016;35:5699–5704. doi: 10.1038/onc.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kajiho H, Kajiho Y, Frittoli E, Confalonieri S, Bertalot G, Viale G, et al. RAB2A controls MT1-MMP endocytic and E-cadherin polarized Golgi trafficking to promote invasive breast cancer programs. EMBO Rep. 2016;17:1061–1080. doi: 10.15252/embr.201642032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang YC, Su CY, Chen MH, Chen WS, Chen CL, Hsiao M. Secretory RAB GTPase 3C modulates IL6-STAT3 pathway to promote colon cancer metastasis and is associated with poor prognosis. Mol. Cancer. 2017;16:135. doi: 10.1186/s12943-017-0687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang J, Liu W, Lu X, Fu Y, Li L, Luo Y. High expression of small GTPase Rab3D promotes cancer progression and metastasis. Oncotarget. 2015;6:11125–11138. doi: 10.18632/oncotarget.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Do MT, Chai TF, Casey PJ, Wang M. Isoprenylcysteine carboxylmethyltransferase function is essential for RAB4A-mediated integrin beta3 recycling, cell migration and cancer metastasis. Oncogene. 2017;36:5757–5767. doi: 10.1038/onc.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mendoza P, Ortiz R, Diaz J, Quest AF, Leyton L, Stupack D, et al. Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells. J. Cell Sci. 2013;126:3835–3847. doi: 10.1242/jcs.119727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diaz J, Mendoza P, Ortiz R, Diaz N, Leyton L, Stupack D, et al. Rab5 is required in metastatic cancer cells for Caveolin-1-enhanced Rac1 activation, migration and invasion. J. Cell Sci. 2014;127:2401–2406. doi: 10.1242/jcs.141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Silva P, Mendoza P, Rivas S, Diaz J, Moraga C, Quest AF, et al. Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget. 2016;7:29548–29562. doi: 10.18632/oncotarget.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J. Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mizuta R, LaSalle JM, Cheng HL, Shinohara A, Ogawa H, Copeland N, et al. RAB22 and RAB163/mouse BRCA2: proteins that specifically interact with the RAD51 protein. Proc. Natl Acad. Sci. USA. 1997;94:6927–6932. doi: 10.1073/pnas.94.13.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manshouri R, Coyaud E, Kundu ST, Peng DH, Stratton SA, Alton K, et al. ZEB1/NuRD complex suppresses TBC1D2b to stimulate E-cadherin internalization and promote metastasis in lung cancer. Nat. Commun. 2019;10:5125. doi: 10.1038/s41467-019-12832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu MH, Luo Y, Qin SL, Zhong M. Increased expression of Rab5A predicts metastasis and poor prognosis in colorectal cancer patients. Int J. Clin. Exp. Pathol. 2015;8:6974–6980. [PMC free article] [PubMed] [Google Scholar]
- 117.Xu W, Shi Q, Qian X, Zhou B, Xu J, Zhu L, et al. Rab5a suppresses autophagy to promote drug resistance in cancer cells. Am. J. Transl. Res. 2018;10:1229–1236. [PMC free article] [PubMed] [Google Scholar]
- 118.Vestre K, Kjos I, Guadagno NA, Borg Distefano M, Kohler F, Fenaroli F, et al. Rab6 regulates cell migration and invasion by recruiting Cdc42 and modulating its activity. Cell Mol. Life Sci. 2019;76:2593–2614. doi: 10.1007/s00018-019-03057-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alonso-Curbelo D, Riveiro-Falkenbach E, Perez-Guijarro E, Cifdaloz M, Karras P, Osterloh L, et al. RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell. 2014;26:61–76. doi: 10.1016/j.ccr.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 120.Yoon SO, Shin S, Mercurio AM. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res. 2005;65:2761–2769. doi: 10.1158/0008-5472.CAN-04-4122. [DOI] [PubMed] [Google Scholar]
- 121.Gundry C, Marco S, Rainero E, Miller B, Dornier E, Mitchell L, et al. Phosphorylation of Rab-coupling protein by LMTK3 controls Rab14-dependent EphA2 trafficking to promote cell:cell repulsion. Nat. Commun. 2017;8:14646. doi: 10.1038/ncomms14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J. Natl Cancer Inst. 2010;102:866–880. doi: 10.1093/jnci/djq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ye B, Duan B, Deng W, Wang Y, Chen Y, Cui J, et al. EGF Stimulates Rab35 activation and gastric cancer cell migration by regulating DENND1A-Grb2 complex formation. Front. Pharm. 2018;9:1343. doi: 10.3389/fphar.2018.01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Novo D, Heath N, Mitchell L, Caligiuri G, MacFarlane A, Reijmer D, et al. Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat. Commun. 2018;9:5069. doi: 10.1038/s41467-018-07339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.