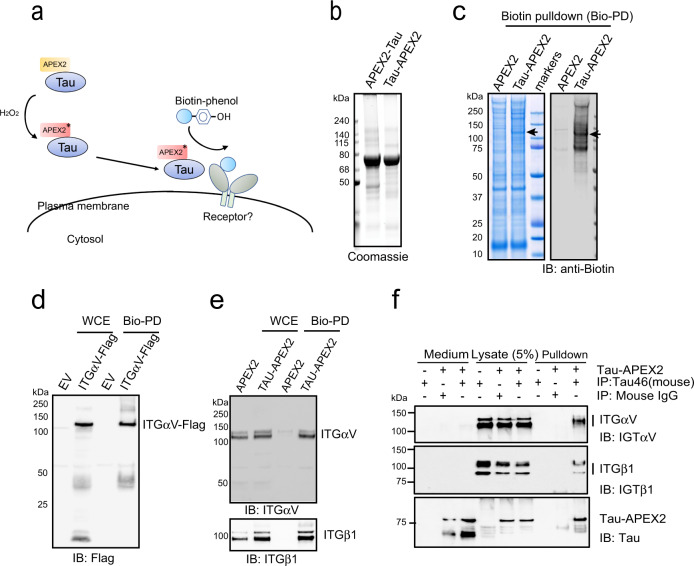
Fig. 1. Identification of integrin αV/β1 as a Tau interactor in primary astrocytes.
a Schematic overview of the proximity-based proteomic mapping strategy. Tau-APEX2 (200 nM) is bound to the cell surface. Biotinylation was initiated by incubating cells with biotin-phenol (BP) in the presence of H2O2 for 4 min. Biotinylated proteins were then purified by affinity chromatography using streptavidin resin. b Coomassie blue staining of purified Tau proteins. c Coomassie blue staining (left) and immunoblotting (IB) (right) of biotinylated proteins from primary astrocytes incubated with APEX2 or Tau-APEX2. The arrow indicates a 120 kDa protein detected in Tau-APEX2-treated cells. d, e Validation of Tau-APEX2-mediated biotinylation of ITGαV/β1. d Tau-APEX2 was incubated with HEK293T cells transfected with either an empty vector (EV) or ITGαV-Flag. Whole cell extracts (WCE) were either directly analyzed by immunoblotting or first subjected to biotin pulldown (Bio-PD) before immunoblotting. e Whole cell extracts (WCE) from primary astrocytes treated with either APEX2 or Tau-APEX2 were analyzed directly by immunoblotting or first subjected to biotin pulldown before immunoblotting. f Co-immunoprecipitation of Tau-APEX2 with ITGαVβ1. Astrocytes were treated with Tau-APEX2 (200 nM) or PBS as a control for 30 min on ice. Cells were lysed in an NP40-containing lysis buffer. Cell lysates were subjected to immunoprecipitation with either a control IgG or a Tau specific antibody (Tau-46). Both cell lysates and precipitated proteins were analyzed by immunoblotting. Where indicated, a fraction of the remaining medium was also analyzed by immunoblotting to show input.