Abstract
Obesity is associated with increased risk of breast cancer. Leptin is a well-known factor involved in obesity and its serum levels are increased in breast cancer. Hyperglycemia is another significant risk factor for breast cancer. Consistently, high glucose induces proliferation and invasion of breast cancer cells and in-vivo calorie restriction reduce tumorigenesis in rodent models. The aim of this study was to investigate the effect of leptin on the viability and mode of cell death in breast cancer cells incubated in different glucose concentrations to represent caloric restriction. For this purpose, MCF-7 and T47D breast cancer cells incubated in different glucose concentrations for a total of 72 hours were treated with or without leptin either for one hour or 24 hours and the ratio of apoptotic, necrotic and alive cells were analyzed by flow cytometry. Our data revealed that glucose incubation significantly decreased apoptosis and necrosis, while increasing viability in both cell lines in a dose dependent manner. One-hour leptin treatment significantly decreased viability, and increased apoptosis but did not significantly affect necrosis in T47D cells incubated in 2.5 mM glucose. In MCF-7 cells, one-hour leptin incubation significantly increased necrosis but its effects on apoptosis and viability were not significant. In conclusion, although glucose induces cell death by apoptosis and necrosis in T47D and MCF-7 cells respectively in a dose dependent manner, the overallviability is still increased in both cell lines. One-hour leptin treatment reverses the effect of low glucose incubation on apoptosis of T47D and necrosis of MCF-7 cells. Moreover, the effect of one-hour leptin treatment on apoptosis or necrosis is significantly higher than that of 24-hour leptin treatment.
Keywords: Calorie restriction, Cancer cell viability, Glucose, Leptin, MCF-7, T47D
1. Introduction
Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer related death among women worldwide (Niu et al., 2013). A number of meta-analyses revealed that obesity and body weight (BW) gain are associated with an increased breast cancer risk, especially for post-menopausal women (Keum et al., 2015). Obesity has been reported to be related to an increased risk of disease recurrence (Ligibel, 2011) and a poorer survival rate (Chan et al., 2014) in both pre- and post-menopausal women diagnosed with breast cancer. Although there are several previous studies investigating the association between obesity and breast cancer development, the molecular mechanism of the association remains largely unknown (Dogan et al., 2007; Dogan et al., 2011; Tuna et al., 2017). In this context, adipokines are suggested as important molecules linking obesity to breast cancer (Dogan et al., 2010; Cicekdal et al., 2019; Cicekdal et al., 2019). Leptin, a 16 kDa peptide hormone, is an adipokine secreted mainly by the adipose tissue as a product of the obese (Ob) gene. There is a well-established link between leptin and obesity (Artac and Altundag, 2012) as leptin is a key appetite-regulating hormone which plays critical roles in appetite control, energy intake and energy expenditure (Amitani et al., 2013). Both leptin and its receptors were reported to be overexpressed in breast cancer tissue (Ishikawa et al., 2004). Consistently, serum leptin levels have also been reported to be significantly higher in breast cancer patients compared to that of healthy individuals (Wu et al., 2009). Besides, circulating leptin levels are higher in breast cancer patients with lymph node metastasis relative to the benign disease breast cancer patients (Niu et al., 2013). Effects of leptin on the proliferation and survival of anchorage independent growth of breast cancer cells have also been demonstrated in vitro (Hu et al., 2002).
Calorie restriction, defined as reducing the energy intake without causing malnutrition, is one of the most accepted preventive approaches for breast cancer development in many species (Dogan et al., 2010; O’Flanagan et al., 2017). In vitro cell culture models may allow for a more controllable calorie intake and also provide quantification of inputs and outputs on a molecular level in a shorter period of time. Therefore, in vitro cell culture experimental design of calorie intake (lower or higher), which is controlled by the glucose concentration in cell culture, may lead to a better understanding of the association between leptin and breast cancer cell proliferation.
Leptin plays an important role in the regulation of glucose metabolism independent of its effect on BW. Inadequate leptin signaling has a pivotal role in hyperglycemia induction, persistence of which may lead to diabetes (Amitani et al., 2013). Consistently, chronic hyperglycemia is a common characteristic of mice lacking the leptin encoding gene (ob/ob) (D’souza et al., 2017) and is alleviated by administration of low doses of leptin that do not significantly affect BW in ob/ob mice (Pelleymounter et al., 1995; Hedbacker et al., 2010). However, not much is known about the effect of leptin on the viability and mode of cell death in breast cancer cells especially in the presence of different glucose concentrations in-vivo or in vitro.
Here, we investigated the effect of leptin on viability and cell death using MCF-7 and T47D breast cancer cell lines cultured in different glucose concentrations (0 mM, 2.5 mM, 5 mM (normal glucose levels in cell medium), 25 mM, 50 mM), which mimics in-vivo calorie intake conditions (lower or higher) in-vitro condition. The present study is the continuation of our previous research which was focusing on the effects of glucose or leptin on cancer cell growth either in-vitro or in-vivo conditions (Cicekdal et al., 2019; Demirel et al., 2019; Tuna et al., 2020). To the best of our knowledge, this is the first study to report the effects of leptin on breast cancer cell viability and mode of cell death in the presence of different glucose concentrations in-vitro.
2. Materials and methods
Cell culture
Estrogen receptor (ER) positive breast cancer cell lines (T47D, MCF-7) were obtained from the American Type Culture Collection (ATCC, VA, USA). These two cell lines differ by their TP53 characteristics. MCF-7 cells have wild type TP53 while T47D cells have mutant TP53 (Neve et al., 2006). Breast cancer cell lines (MCF-7 and T47D) were chosen to be studied because the focus of our group has been breast cancer and obesity in mouse models (Dogan et al., 2007; Demirel et al., 2019). These two cells lines have different characteristics for intracellular apoptotic signaling proteins. Although T47D cells have caspase 3 activity, MCF-7 cells don’t have caspase 3 activity, suggesting that leptin may be triggering a different apoptotic pathway(s) (Mooney et al., 2002). Cells were grown in complete DMEM medium (Gibco, NY, USA) containing 5 mM glucose, 10% heat inactivated FBS (Gibco, NY, USA), 0.25% Insulin (Gibco, NY, USA), 1% Pen/Strep (Gibco, NY, USA) then incubated in 5% CO2 at 37°C until they reached 70% confluency prior to the experiments.
Experimental design
After reaching confluency, T47D (passage 29) and MCF-7 (passage 19) cells were washed with PBS and then trypsinized at 37°C to collect cells in normal media. Cells were centrifuged at 1000 rpm for 7–10 min, resuspended in medium, an equal number of cells (8.5 × 105 cells/plate) were placed in petri dishes and incubated overnight to allow the cells to attach. On the following day, cell medium was removed and replaced with fresh DMEM containing differing amounts of glucose (0 mM, 2.5 mM, 5 mM, 25 mM, and 50 mM) and incubated at 37°C for 72 hours. Normal glucose concentration in a cell medium is 5 mM. Therefore, two of the glucose concentrations tested were below the normal glucose concentration (0 mM, 2.5 mM,) while the other two concentrations were above the normal glucose levels (25 mM, 50 mM). To see the effects of leptin on breast cancer cells in different glucose concentrations, 100 ng/ml leptin (Cell Sciences, MA, USA) was added to the cell medium either at the end of either 48 or 71 hours after the start of incubation of the cells with the medium containing different glucose concentration. Cells were further incubated in medium with or without leptin until 72 hours from the start of glucose incubation. In other words, the cells were incubated with leptin either for 24 hours or one-hour.
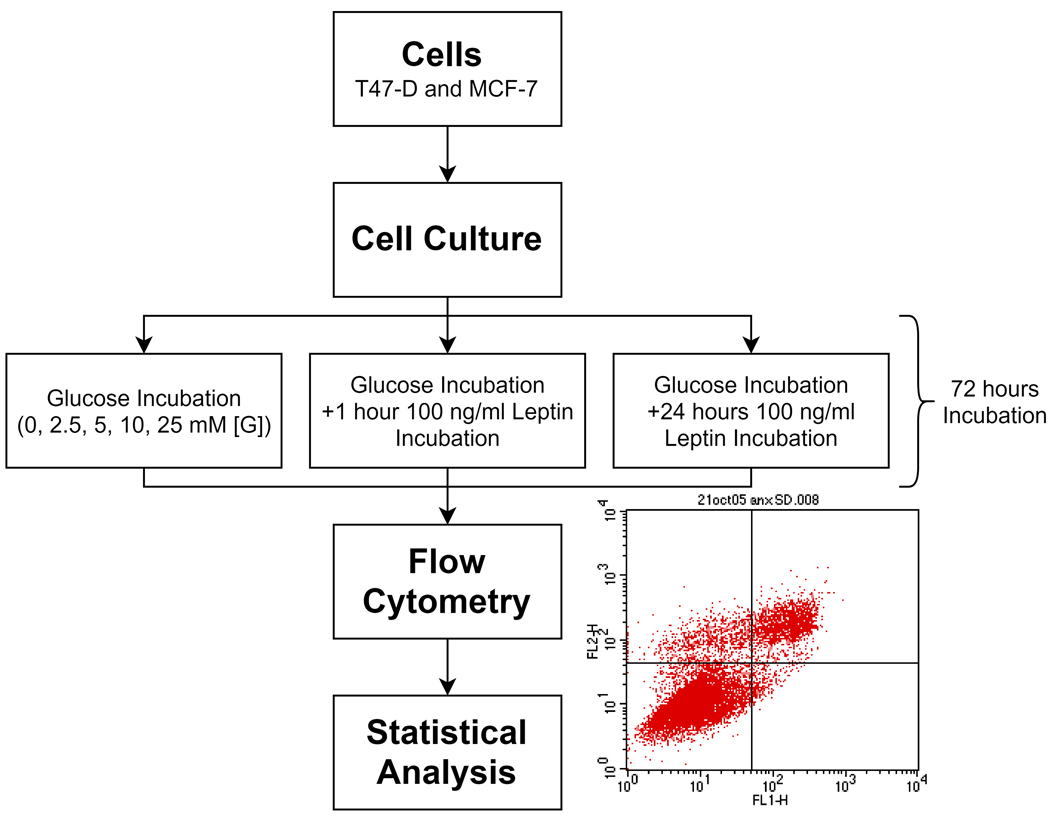
Thus, total glucose (Sigma, MO, USA) incubations were 72 hours at each glucose concentration tested (either 48 hours glucose incubation +/− 24-hour leptin treatment or 71 hours glucose incubation +/− one-hour leptin treatment). Study design is shown in Fig. 1. For each set of experiments on a given day, two to three different petri dishes were used for each glucose concentration and the average of these replicates was taken as one sample (duplicate or triplicate).
Fig. 1.
Experimental study design.
Flow cytometry analysis of apoptosis
Following the 72 hour-incubations, T47D or MCF-7 cells were trypsinized (Gibco, NY, USA) and harvested. Cells were then centrifuged, washed with PBS and labeled with Annexin V-Fluorescein Isothiocyanate and PI (Propidium Iodide) (Annexin V-FITC Apoptosis Detection Kit, MBL International Corp., MA, USA). The distribution pattern of alive and dead (apoptotic/necrotic) cells were determined by FACS analysis (Becton-Dickinson FACS Calibur flow cytometer, BD Biosciences, CA, USA). The FITC and PI signals were detected in FL1 and FL2 channels, respectively. Small debris were excluded by gating intact cells in the FSC/SSC plot. Cells in the lower left panel of the FL1/FL2 dot plot (labeled with low Annexin V-FITC and PI) were considered as viable, cells in the right panel (labeled with Annexin V-FITC) were considered to be apoptotic, and cells in the upper left plot (labeled with PI only) were considered to be in necrosis.
Statistical analysis
Two tailed Mann Whitney test and Kruskal-Wallis test with Dunn’s multiple comparison test were performed to determine statistical differences among the groups. All analyses were conducted in Graphpad Prism 7.0. Data represent mean ± standard deviation (SD). Differences were accepted as statistically significant when p<0.05. “n value” for each comparison is given in the figure legends.
3. Results
Glucose decreases cell death in T47D and MCF-7 cells in a dose dependent manner
MCF-7 and T47D cells were incubated in medium containing different glucose concentrations for 72 hours and necrotic/apoptotic cells were analyzed using flow cytometry to investigate the effect of glucose on breast cancer cell death (Fig. 2). All tested glucose concentrations (2.5 mM, 5 mM, 25 mM, and 50 mM) significantly decreased cell death in both T47D and MCF-7 cells compared to the glucose free group (p<0.05, Fig. 2). Although incubation in each glucose concentration led to a significant decrease in apoptosis in both T47D and MCF-7 cells (p<0.05, Fig. 2A, 2B), the levels of apoptosis in T47D cells were lower than that of MCF-7 cells for 2.5 mM glucose concentration (p<0.0001 for T47D cells, p<0.05 for MCF-7 cells).
Fig. 2.
Effect of different glucose concentrations on cell death in breast cancer cells. A) Apoptosis of T47D (n=30) B) Apoptosis of MCF-7 (n=29) cultured in 0 mM, 2.5 mM, 5 mM, 25 mM, or 50 mM glucose. C) Necrosis of T47D (n=29–31) D) Necrosis of MCF-7 (n=29) cultured in 0 mM, 2.5 mM, 5 mM, 25 mM, or 50 mM glucose for 72 hours. n represents the number of independent experiments. Data represent mean ± S.D. *, p<0.05, **** p<0.0001.
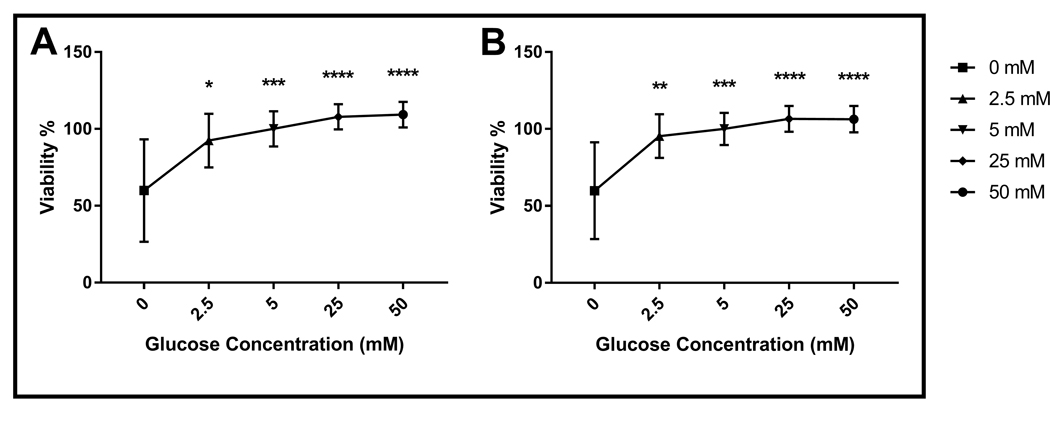
All glucose concentrations significantly decreased necrosis compared to 0 mM glucose except for 2.5 mM in T47D cells (p<0.05, Fig. 1C). On the other hand, all glucose concentrations decreased necrosis significantly compared to the 0 mM glucose group in MCF-7 cells (p<0.0001, Fig. 2D). However, the effect of 5 mM glucose on necrosis was more drastic in MCF-7 cells compared to T47D cells (p<0.0001 for MCF-7 cells, p<0.05 for T47D cells, Fig. 1D). Consistently, glucose incubation significantly increased viability of both MCF-7 and T47D cell lines in a dose dependent manner (p<0.05, Fig. 3). Notably, the positive effect of 2.5 mM glucose incubation on viability was more apparent in MCF-7 cells compared to T47D (p<0.01 for MCF-7 cells, p<0.05 for T47D cells, Fig. 3).
Fig. 3.
Effect of different glucose concentrations on the viability of breast cancer cells. A) Viability of T47D (n=30) and B) Viability of MCF-7 (n=28) cultured in various glucose concentrations for 72 hours. n represents the number of independent experiments. Data represent mean ± S.D. *, p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Leptin enhances the effect of glucose on cell death in T47D and MCF-7 cells
In order to examine the effects of leptin on the viability of breast cancer cells cultured in medium with different glucose concentrations, T47D and MCF-7 cells incubated in 0 mM, 2.5 mM, 5 mM, 25 mM, or 50 mM glucose for 72 hours in total were treated with or without leptin (100 ng/ml) for either one-hour or 24 hours and necrotic and apoptotic cells were analyzed by flow cytometry (Fig. 4). One-hour leptin incubation significantly increased apoptosis (p<0.05, Fig. 4A), but did not dramatically affect necrosis in T47D cells cultured in medium with 2.5 mM glucose (p>0.05, Fig. 4B). Consistently, viability was significantly lower in one-hour leptin treated cells cultured in 2.5 mM glucose compared to leptin untreated cells cultured in 2.5 mM glucose (p<0.05, Fig. 4C). Notably, the positive effect of one-hour leptin treatment on the apoptosis of T47D cells in 2.5 mM glucose was significantly higher compared to 24-hours leptin treatment (p<0.05, Fig. 4A). However, the effect(s) of one-hour leptin treatment on the necrosis of T47D cells was not significantly different compared to the 24-hour leptin treatment (p>0.05, Fig. 4B). In support of these data, viability was significantly decreased in T47D cells incubated in medium with 2.5 mM glucose and then treated with leptin for one-hour compared to 24-hour leptin treatment (p<0.01, Fig. 4C). Similar to 2.5 mM glucose cultured cells, one-hour leptin treatment significantly increased apoptosis in T47D cells in 5 mM glucose (p<0.05, Fig. 4A) but, it did not have a significant effect on necrosis in T47D cells incubated in 5 mM glucose (p>0.05, Fig. 4B). Although not statistically significant, one-hour leptin treatment decreased viability of T47D cells incubated in 5mM glucose (p>0.05, Fig. 4C). Besides, compared to the 24-hour leptin treatment, the negative effect of one-hour leptin treatment on viability of T47D cells incubated in either 2.5 mM or 5mM glucose was significantly higher (p<0.05, Fig. 4C).
Fig. 4.
Effect of leptin on the viability of T47D cells cultured in different glucose concentrations. A) Apoptosis of T47D B) Necrosis of T47D C) Viability of T47D cultured in different glucose concentrations in the presence or absence of leptin (100 ng/ml) (n=7–29). n represents the number of independent experiments. Data represent mean ± S.D. * p<0.05, ** p<0.01.
Unlike T47D cells, one-hour leptin treatment significantly increased necrosis in MCF-7 cells (p<0.001, Fig. 5B), but did not have a significant effect on apoptosis in 2.5 mM glucose (Fig. 5A). Although not statistically significant, viability of one-hour leptin treated MCF-7 cells cultured in 2.5 mM glucose was lower compared to leptin untreated cells (p>0.05, Fig. 5C). The positive effects of one-hour leptin treatment on necrosis of MCF-7 cells incubated in either 2.5 mM or 5 mM glucose were significantly higher compared to 24-hour leptin treated cells (p<0.05, Fig. 5B), although apoptosis was not significantly different in these groups (p>0.05, Fig. 5A). Consistently, one-hour leptin treatment significantly decreased viability of MCF-7 cells in either 2.5 mM or 5 mM glucose relative to the 24-hour leptin treatment (p<0.01, Fig. 5C). Compared to leptin untreated cells, one-hour leptin treatment significantly (p<0.05) reduced viability of MCF-7 cells incubated in 5 mM glucose, however, it did not have a significant effect on either necrosis or apoptosis (Fig. 5). One or 24-hour leptin treatment did not have any significant effect on modes of cell death or viability in either MCF-7 or T47D cells (Figs. 4, 5).
Fig. 5.
Effect of leptin on the viability of MCF-7 cells cultured in different glucose concentrations. A) Apoptosis of MCF-7 B) Necrosis of MCF-7 C) Viability of MCF-7 cultured in different glucose concentrations in the presence or absence of leptin (100 ng/ml) (n=4–30). n represents the number of independent experiments. Data represent mean ± S.D. *, p<0.05, ** p<0.01, *** p<0.001.
Our data showed that glucose significantly decreased cell death while increasing viability in both MCF-7 and T47D cell lines in a dose dependent manner. Additionally, one-hour leptin treatment significantly increased apoptosis in T47D cells cultured in the presence of 2.5 mM glucose, however it significantly increased necrosis in MCF-7 cells. Moreover, one-hour leptin treatment increased necrosis more significantly in 5 mM glucose cultured MCF-7 cells relative to T47D cells. Besides, the effect of one-hour leptin treatment of MCF-7 and T47D cells cultured in low (2.5 mM) and physiological (5 mM) doses of glucose was more significant compared to that of 24-hour leptin treatment.
4. Discussion
Hyperglycemia, defined as increased glucose levels in blood, is a significant risk factor for breast cancer development especially for post-menopausal women (Boyle et al., 2012). Several studies have suggested that hyperglycemia may contribute to cell proliferation, apoptosis, metastasis, and chemotherapy resistance of cancer cells (Vigneri et al., 2009; Johnson et al., 2012; Duan et al., 2014; Ryu et al., 2014; Demirel et al., 2019). Consistently, glucose and other factors involved in glucose metabolism including insulin and insulin-like growth-factors (IGFs) contribute to breast cancer development (Muti et al., 2002; Dogan et al., 2011). In addition, incubation in high glucose containing medium induced proliferation and invasion of breast cancer cells in-vitro (Wei et al., 2017; Demirel et al., 2019). On the other hand, leptin, a key appetite-regulating hormone, is recognized as another important risk factor for breast cancer development and prognosis since both leptin and its receptors are reported to be overexpressed in breast cancer (Ishikawa et al., 2004). In this study, we examined the effect of leptin on cell death and viability in T47D and MCF-7 breast cancer cell lines cultured in cell media with different glucose concentrations to mimic leptin’s effect on calorie intake (lower or higher) in-vitro.
Although there are studies performed in high glucose medium and assessing cell proliferation, to our knowledge there are no previous studies investigating the effects of leptin at different glucose concentrations, specifically lower than normal glucose concentration (5 mM glucose) in cell media. In this context, high glucose levels were previously demonstrated to promote proliferation of breast cancer cells (Yamamoto et al., 1999; Okumura et al., 2002; Hou et al., 2017; Wei et al., 2017; Demirel et al., 2019). Consistently, we showed that glucose increases viability and decreases cell death in both T47D and MCF-7 cells in a dose dependent manner (Figs. 2 ,3). Specifically, high glucose concentrations (25 mM and 50 mM) decrease necrosis and apoptosis significantly in both MCF-7 and T47D cell lines. However low glucose (2.5 mM), which was used to mimic calorie restriction in-vitro, significantly induced apoptosis in T47D and necrosis in MCF-7 cells. Additionally, physiological normal glucose (5 mM) increases necrosis more significantly in MCF-7 cells compared to T47D cells (Fig. 2A, 2B). In this context, although Krętowski et al. reported a decrease in the percentage of apoptotic cells in low glucose (2.8 mM) compared to the high glucose concentration of 25 mM (Krętowski et al., 2016), our data indicated that apoptosis was lower in high glucose cultured cells (25 mM and 50 mM) compared to low glucose (2.5 mM) in both MCF-7 and T47D cells (Figs. 2, 3). A major difference between Krętowski et al.’s study and ours that might have contributed to the different findings is in the duration of the glucose incubations: Krętowski et al. examined the apoptotic cells following a 48-hour incubation, whereas we applied a 72-hour glucose incubation prior to examining apoptosis.
Okumura et al., previously reported that 5 day-treatment of 10–9 or 10–8 M leptin in the presence of physiological glucose concentration (5.5 mM) did not significantly increase proliferation of MCF-7 cells (Okumura et al., 2002). Consistently, we also did not observe a significant effect on the viability of MCF-7 cells following the 24-hour treatment of 6.25×10–9 M leptin. However, one-hour leptin (6.25×10–9 M) treatment significantly decreased viability in 5 mM glucose-cultured MCF-7 cells, indicating that leptin might have different effects on the viability of MCF-7 cells dependent on the dose and duration of the treatment. Other previous studies have also reported different results regarding hyperleptinemia both in-vitro and in-vivo studies. One of the reasons for this could be leptin resistance effects especially in obese subjects.
Our data revealed that glucose incubation significantly reduced apoptosis and necrosis, while increasing overall viability in the T47D cell line in a dose dependent manner. Additionally, one-hour leptin treatment of T47D cells significantly increased apoptosis in 2.5 mM glucose. In MCF-7 cells, glucose incubation decreased both apoptosis and necrosis in a dose dependent manner, thus increasing overall viability. One-hour leptin treatment significantly increased necrosis in 2.5 mM glucose cultured MCF-7 cells. In both cell lines, the effect of one-hour leptin treatment on apoptosis or necrosis was significantly higher compared to 24-hour leptin treatment. It’s noteworthy that leptin at low and physiological glucose concentrations increase apoptosis in T47D cells which lack P53, suggesting that leptin may be triggering a P53-independent apoptotic pathway(s) (Mooney et al., 2002). Although Okumura et al., reported that 5-day treatment of 10–6 M leptin enhanced proliferation of MCF-7 cells incubated in 25 mM glucose, in the present study we did not observe any significant effect of one or 24-hour 6.25×10–9 M leptin treatment on the viability of T47D and MCF-7 cells incubated in 25 mM or 50 mM glucose compared to 5 mM (Okumura et al., 2002). The different effects of leptin and glucose concentrations on the mechanisms of cell death in T47D and MCF-7 cell lines might be due to the genetic background differences. It is also not unusual to see different responses for leptin and/or glucose in different cell lines. Therefore, these results should be tested in healthy breast cell lines to see whether leptin effects are specific to cancerous cells.
These results reveal that leptin effect for cell viability, apoptosis and necrosis are secondary in the presence of enough levels of glucose in breast cancer cell lines in in-vitro conditions. However, leptin may play a glucose like role in the absence of glucose in cancer cell growth. More mechanistic studies are needed to clarify the cross-talk between leptin and glucose signaling pathways in regard to cancer cell proliferation.
Acknowledgements
This work was supported by grants NIH-CA101858, CA157012 (MPC) and The Breast Cancer Research Foundation and The Hormel Foundation, Minnesota. The authors thank summer intern undergraduate student, Swetha S. Ramanan, for helping us maintaining the cell culture.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- Amitani M, Asakawa A, Amitani H, Inui A, 2013. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 7, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artac M, Altundag K, 2012. Leptin and breast cancer: An overview. Med. Oncol. 29, 1510–1514. [DOI] [PubMed] [Google Scholar]
- Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, Fairley LL, Boniol M, Zheng T, Zhang Y, Pasterk M, Smans M, Curado MP, Mullie P, Gandini S, Bota M, Bolli GB, Rosenstock J, Autier P, 2012. Diabetes and breast cancer risk: A meta-analysis. Br. J. Cancer. 107, 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, Mc Tiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T, 2014. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 25, 1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicekdal MB, Tuna BG, Charehsaz M, Cleary MP, Aydin A, Dogan S, 2019. Effects of long term intermittent versus chronic calorie restriction on oxidative stress in a mouse cancer model. IUBMB Life. 71, 1973–1985. [DOI] [PubMed] [Google Scholar]
- Cicekdal MB, Kazan BT, Tuna BG, Ozorhan U, Ekici ID, Zhu F, Suakar O, Kuskucu A, Bayrak OF, Arcaro K, Cleary MP, Atasayan O, Yılmaz B, Dogan S, 2019. Effects of two types of calorie restriction on methylation levels of adiponectin receptor 1 (adipor1) and leptin receptor overlapping transcript (leprot) in a MMTV-TGF-α breast cancer mouse model. Br. J. Nutr. 5, 1–23. [DOI] [PubMed] [Google Scholar]
- Demirel PB, Ozorhan U, Tuna BG, Cleary MP, Dogan S, 2019. Effects of different glucose concentrations on the leptin signaling pathway in MCF-7 and T47D breast cancer cells. Ann. Med. Res. 12, 2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ, 2017. The glucoregulatory actions of leptin. Mol. Metab. 6, 1052–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP, 2007. Effects of high fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res. 9, R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan S, Johannsen AC, Grande JP, Cleary MP, 2011. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-I signaling pathways in mammary fat pad tissues and mammary tumors. Nutr. Cancer. 63, 389–401. [DOI] [PubMed] [Google Scholar]
- Dogan S, Rogozina OP, Lokshin AE, Grande JP, Cleary MP, 2010. Effects of chronic vs. intermittent calorie restriction on mammary tumor incidence and serum adiponectin and leptin levels in MMTV-TGF-alpha mice at different ages. Oncol. Lett. 1, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Shen X, Lei J, Xu Q, Yu Y, Li R, Wu E, Ma Q, 2014. Hyperglycemia, a neglected factor during cancer progression. Biomed. Res. Int. 2014, 461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM, 2010. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 11, 11–22. [DOI] [PubMed] [Google Scholar]
- Hou Y, Zhou M, Xie J, Chao P, Feng Q, Wu J, 2017. High glucose levels promote the proliferation of breast cancer cells through GTPases. Breast Cancer (Dove Med. Press). 9, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Juneja SC, Maihle NJ, Cleary MP, 2002. Leptin-a growth factor in normal and malignant breast cells and for normal mammary gland development. J. Natl. Cancer Inst. 94, 1704–1711. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Nagawa H, 2004. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 10, 4325–4331. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG, 2012. Diabetes and cancer (1): Evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 55, 1607–1618. [DOI] [PubMed] [Google Scholar]
- Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL, 2015. Adult weight gain and adiposity-related cancers: A dose-response meta-analysis of prospective observational studies. J. Natl. Cancer Inst. 107. [DOI] [PubMed] [Google Scholar]
- Kretowski R, Borzym-Kluczyk M, Stypulkowska A, Branska-Januszewska J, Ostrowska H, Cechowska-Pasko M, 2016.Low glucose dependent decrease of apoptosis and induction of autophagy in breast cancer MCF-7 cells. Mol. Cell Biochem. 417, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligibel J, 2011. Obesity and breast cancer. Oncology (Williston Park). 25, 994–1000. [PubMed] [Google Scholar]
- Mooney LM, Al-Sakkaf KA, Brown BL, Dobson PR, 2002. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br. J. Cancer. 87, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schünemann HJ, Ram M, Freudenheim JL, Sieri S, Trevisan M, Berrino F, 2002. Fasting glucose is a risk factor for breast cancer: A prospective study. Cancer Epidemiol. Biomarkers Prev. 11, 1361–1368. [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW, 2006. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 10, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L, 2013. The association between leptin level and breast cancer: A meta-analysis. PLoS One. 8, e67349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flanagan CH, Smith LA, McDonell SB, Hursting SD, 2017. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 15, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M, Yamamoto M, Sakuma H, Kojima T, Maruyama T, Jamali M, Cooper DR, Yasuda K, 2002. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: Reciprocal involvement of PKC-alpha and PPAR expression. Biochim. Biophys. Acta. 1592, 107–116. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F, 1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 269, 540–543. [DOI] [PubMed] [Google Scholar]
- Ryu TY, Park J, Scherer PE, 2014. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 38, 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuna BG, Atalay PB, Altunbek M, Kalkan BM, Dogan S, 2017. Effects of chronic and intermittent calorie restriction on adropin levels in breast cancer. Nutr. Cancer. 69, 1003–1010. [DOI] [PubMed] [Google Scholar]
- Tuna BG, Cleary MP, Demirel PB, Dogan S, 2020. Leptin signaling in liver tissue of a transgenic breast cancer mouse model. Cureus. 12, e6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R, 2009. Diabetes and cancer. Endocr. Relat. Cancer. 16, 1103–1123. [DOI] [PubMed] [Google Scholar]
- Wei ML, Duan P, Wang ZM, Ding M, Tu P, 2017. High glucose and high insulin conditions promote MCF7 cell proliferation and invasion by upregulating IRS1 and activating the Ras/Raf/ERK pathway. Mol. Med. Rep. 16, 6690–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, Yu JC, Sun CA, 2009. Circulating levels of leptin, adiposity and breast cancer risk. Br. J. Cancer. 100, 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Patel NA, Taggart J, Sridhar R, Cooper DR, 1999. A shift from normal to high glucose levels stimulates cell proliferation in drug sensitive MCF-7 human breast cancer cells but not in multidrug resistant MCF-7/ADR cells which overproduce PKC-beta II. Int. J. Cancer. 83, 98–106. [DOI] [PubMed] [Google Scholar]