Abstract
The photodegradation of 2-chlorobenzoic acid (2-CBA) in suspensions of TiO2 was examined under different operational parameters. The optimal condition could be obtained through the experiment, i.e. that the concentration of 2-CBA was 30 mg/L and the dosing quantity of TiO2 was 0.01 g under UV light in the case of pH 3.5. Above reaction process was in accordance with first order kinetics model. The influence on photocatalytic degradation caused by typical anions in eutrophicated water body such as NO3− and H2PO4− was explored in this work, which revealed that both two anions had inhibitory effect on the degradation process. In addition, alcohol was introduced into the process to identify the degradation mechanism of 2-CBA with TiO2, and the reaction route of 2-CBA could be predicted through the analysis on the intermediate.
Keywords: Degradation of 2-CBA, Eutrophicated water, Photocatalytic
1. Introduction
Recently chlorobenzoic acid (CBA) was widely used into every aspect of our life, such as industry, agriculture, pharmaceutical and so on (Cassani et al., 2013, Blackledge et al., 2000). CBA is an important organic compound with good water-soluble which may cause persistent pollution in the aquatic environment and it is harmful to human body and can also lead to macrocephaly, cancer and mutation (Gichner et al., 2008). In addition, it was difficult to achieve the effective degradation of CBA by conventional biological treatment. There were some reports about the degradation of the CBA. Li et al. researched the degradation of p-CBA using nickel modified activated carbon in ozone atmosphere and the removal rate of TOC (Total Organic Carbon) can reach to 60% (Li et al., 2009). B. Neppolian et al. used ultrasound/FeOOH-H2O2 to realize degradation of p-CBA. It was found to be substantially faster than that with ultrasound only (Neppolian et al., 2004). Milan Muziká et al. used ligninolytic fungal strains to degrade CBA, and the removal rate in the soil was almost 99% within 60 days (Muzikář et al., 2011). Asma Mhemdi et al. used electric-Fenton to degrade 2-CBA, in which the removal rate of TOC almost reaches to 80% 3 h later (Mhemdi et al., 2013).
A lot of literatures had described how to use advanced oxidation to degrade CBA, but there were much few articles to research the degradation of CBA with TiO2 photocatalytic technology. It is well known that TiO2 with cheapness, non-toxic, harmlessness, high chemical stability is widely used in the manufacture of products and the environment treatment such as surface cleaning products, air and water self-purification, photocatalysis, et al. (Nakata and Fujishima, 2012, Pelaez et al., 2012).
Elsevier B.V. et al. employed low temperature plasma combined with TiO2 to eliminate isovaleraldehyde from the air at 364 nm, the results showed that isovaleraldehyde conversion reached 85% (Maciuca et al., 2012). L. Prieto-Rodriguez et al. use TiO2 and H2O2 in the sun simulator as tertiary treatment for wastewater treatment plants in wastewater. The results showed that when dosing quantity of TiO2 and H2O2 was 50 mg/L and 100 mg/L respectively, sulfamethoxazole in the waste water reduced from 100 mg/L to 65 mg/L Ku et al. (1996). Yu et al. used 0.2 mg/mL S-doped-TiO2 to remove the Gram positive bacterium (Micrococcuslylae) in water with 100 W tungsten halogen lamp, the degradation rate as high as 96.7% after one hour (Yu et al., 2005).
Photocatalytic degradation of 2-CBA in pure water would be markedly different from that in contaminated water. Angela-Guiovana Rincón found that the bacterial inactivation rate had a meaningful decrease when various anios were introduced into the process of photocatalytic degradation bacteria with TiO2 (Rincon & Pulgarin, 2004). Liang et al. reported that the existence of Cl−, NO3−, H2PO4−, and SO42− (0.05 M) had an obvious negative impact on the degradation of DCP (20 mg/L) when pH value was 5.3. The results showed that the removal rate of 2, 3-DCP without anion was 93%, but it was only 77% when the concentration of Cl− was 0.05 M (Liang et al., 2008). All of above showed that the existence of anion in water lead a great influence on photodegradation of organic matter. It was found that human nutrient input was a major cause of eutrophication. Eutrophication can lead lack of oxygen of water and a large number of aerobic biological to die (Zhu et al., 2014, Lenhart et al., 2010, Garnier et al., 2005, Liu and Yin, 2010). Liu et al. found that the rate of N/P in Bohai Sea is greater than 16 (Redfield number) in 2004. The simulation and observe showed that algae bloomed in spring nitrogen became a limiting factor and whereas phosphorus limited in summer (Liu & Yin, 2007). Inspired by the finding we will insight into the influence of the ion in eutrophicated water on the photocatalytic process. The effects of N, P on the photocatalytic treatment were explored in this paper by using KNO3 and KH2PO4 as nitrogen and phosphorous source respectively.
2. Materials and methods
2.1. Materials and equipment
2-Chlorobenzoic acid (AR) was pursued from China National Medicine Group. TiO2, Potassium nitrate (KNO3) and Potassium dihydrogen phosphate (KH2PO4) were supplied by Tianjin wind ship chemical technology co., LTD. Bench centrifuge: Shanghai anting scientific instrument factory, TGL-16C. Ultraviolet–Visible spectrophotometer (Robert lieber tyco Beijing instrument co., LTD. Lab-Tech). TOC (The Americas GE Analytical Instruments, Silvers 900)
2.2. Photocatalytic reaction
All reactions were kept at room temperature. 100 mL 2-CBA solution and a certain amount of TiO2 were introduced into a beaker (250 mL). The magnetic stirrer was used to enhance the mass transport of the whole reaction system. First of all, the beaker was put in dark for one hour. Then the UV lamp (λ = 254 nm, 25 W) was turned on after the adsorption equilibrium between 2-CBA and TiO2 had been established, and was kept under the irradiation for 4 h. After reaction, the mixture were taken to centrifuge for 10 min at 12,000 r/min.
2.3. Analytical methods
Concentrations of 2-CBA at different reaction time were determined by ultraviolet–visible spectrophotometer, the absorbance wavelength was 210 nm. Some samples also need to be determined by TOC. Kinetic constants are critical to evaluate the performance of photodegradation reaction. The kinetic constant with the reaction time and reactants concentration have been simulated in this work to analysis the photolysis process.
3. Results and discussion
3.1. Initial concentration
The impact of various concentration of 2-CBA in the reaction system was detected by TOC while the dosing quantity of TiO2 was added. The initial concentration of 2-CBA was 5, 10, 20, 30, 40 mg/L (ppm) respectively. The results were showed in Fig. 1 and Fig. 2.
Fig. 1.
The kinetic analysis of various concentration of 2-CBA.
Fig. 2.
The influence of various concentration on removal rate of 2-CBA.
Initially, the removal rate decreased quickly and then keep stable as the concentration of 2-CBA increased. When the starting content was 5 mg/L, the degradation rate achieved 89.84% after the reaction lasting eight hours. Along with the rising of the starting content, the degradation rate of 2-CBA reached 76.41%, 79.33%, 69.66%, and 74.65% respectively. All the above can be attributed to two reasons: (1) With the raise of the starting content of 2-CBA, solution transmittance was relatively lower which would decrease the quantity of photons accessing to the TiO2 and lead a drop of the photodegradation efficiency; (2) The number of photogenerated hole which generated on the catalyst surface corresponded to the amount of TiO2. The oxidation degradation of organic matter by photogenerated hole was an important way for photodegradation reaction with TiO2 (Lin & Lin, 2014). In this work, the best initial concentration of TiO2 was chosen as 30 mg/L.
3.2. The dosing quantity of catalyst
To analyze the effect of dosing quantity of TiO2 on the efficiency of 2-CBA photodegradation, the account of TiO2 was carried out in the range from 0.005 to 0.025 g.
Fig. 3 showed that removal rate coefficient of 2-CBA raised along with the increase of catalyst when the catalyst dosing quantity was less than 0.01 g. And then the rate constant decreased along with the increase of catalyst. This phenomenon was identified with report by Biljana et al., the degradation rate reached 82% under UV light irradiation for 4 h when dosing quantity of TiO2 was 0.01 g (Abramovic et al., 2013). The photodegradation reaction accords with the first order kinetics model and the catalyst dosing quantity had a great influence on reaction. Both larger and smaller dosing quantity of catalyst would inhibit the degradation, which could due to two reasons. While TiO2 was relatively less, the hydroxyl radicals produced in the reaction system would be little and were unfavorable to removing on organic matter. When the dosing quantity was excessive, TiO2 would agglomerate on the solution surface and hinder the photons from reaching the surface of the TiO2, that would lower photodegradation efficiency. It was observed in Fig. 4 that the optimum amount was 0.01 g.
Fig. 3.
The influence of different dosing quantity TiO2 on degradation of 2-CBA.
Fig. 4.
The kinetic analysis of different dosing quantity on the removal of 2-CBA.
3.3. pH
The initial pH was maintained at 3.5, 4.5, 5.5, 6.5, 7.5 or 8.5 in a suspension of TiO2 (0.01 g/L) and 2-CBA (30 mg/L). Fig. 5 showed the degradation rate of 2-CBA. The kinetic rate constant was summarized in Fig. 6.
Fig. 5.
The effects of pH on the removal rate of 2-CBA.
Fig. 6.
The kinetic analysis of pH on the removal of 2-CBA.
A dramatically decreasing trend of degradation rate of 2-CBA was shown in Fig. 5. It was described in Fig. 6 that the pH value had little effect on the kinetic constants of 2-CBA between 5.5 and 8.5 while the constants declined gradually along with pH value from 3.5 to 5.5. The removal rate of 2-CBA had the maximum at 82.8% when pH value was 3.5. In addition, no matter how pH value changed, the degradation of 2-CBA was coincided with typical first-order kinetic reaction. This result accorded with the relevant report by Rincon (Maciuca et al., 2012).
The pH has significant impact on the zeta potential, which determines the charge characteristics on TiO2 particle surface. The charge turned from positive into negative when chemical condition changed from acidic to alkaline (Haque & Muneer, 2007). While pH of reaction system is greater than pKa value of 2-CBA (2.9), 2-CBA exists in ion state. Therefore, when pH value was lower than 6.5 more 2-CBA ion adsorption on the surface of TiO2, which was favorable to the oxidative degradation. On the contrary, the photodegradation was inhibited by electrostatic repulsion between 2-CBA ion and TiO2 surface charge when pH value was higher than 6.5.
3.4. Anionic effect
3.4.1. Nitrogen
The excessive nutrients into water especially nitrogen and phosphorus would enhance phytoplankton development and destroy the ecological balance. We need to consider the effect of nitrogen and phosphorus on the photodegradation since a lot of rivers and lakes have presented a serious state of eutrophication. The influence of anions in water could be divided into inhibition, promotion and no influence. In Dunia’s report, when Cl− was in solution (300 mg/L), the removal rate of imazalil (50 mg/L) fell nearly 10% than that without Cl−. In Wang’s report, H2O2 (0.06 mol/L) was introduced to evaluate the photodegradation of MO instead of TiO2, and the result showed that 6% disappeared in 12 min. In this work, 0.5, 1, 2, 3, 4, 5 mL KNO3 solution (100 mg/L) was added into reaction system respectively under the optimal conditions (initial concentration is 30 mg/L, pH3.5, catalyst dosing quantity is 0.01 g). In other word, the inorganic nitrogen concentration was set from 0.5 to 5 mg/L (Santiago et al., 2014, Wang et al., 2013). The result was showed in Fig. 7 and Fig. 8.
Fig. 7.
The effect of NO3− on the photodegradation of 2-CBA.
Fig. 8.
The kinetic analysis of NO3− on the degradation of 2-CBA.
Fig. 7 showed that the degradation rate reclined with the increase of KNO3. From the experiment result, NO3− had a negative influence on the photodegradation. The removal rate was only 39% in present of NO3− (5 mg/L) after 3 h, and the degradation efficiency had fallen by nearly 50% compared with the case without NO3−. This result was consistent with the conclusion in other research. But Senthil et al. drawn a opposite conclusion. They used TiO2 to take the photocatalytic degradation on dye in the present of NO3− and the result showed that NO3− had promoted the photocatalytic reaction (Barndõk et al., 2012). Through the kinetics analysis (Fig. 8), it could be found that the removal rate of 2-CBA dropped significantly and then retained steady with the increase of NO3−. The inhibition of photocatalytic activity could due to that NO3− acted as the scavenger of hydroxyl radicals or photogenerated hole. In theory, hydroxyl radicals or photogenerated hole can oxidize organic matter in the reaction system (Antoine et al., 2001). So the presence of NO3− inhibited the photocatalytic ability and had a negative impact on removing 2-CBA.
3.4.2. Effect of phosphorus
Under the best condition of mineralize 2-CBA, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0 mL KH2PO4 (10 mg/L) were added into the solution respectively, and phosphorus concentrations in the solution were 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 mg/L.
Fig. 9 indicated that the performance of phosphorus in the solution was consistent with above mentioned nitrogen. Both ions had negative effect on the photodegradation of 2-CBA. With increase of phosphorus concentration, the removal rate of 2-CBA reduced gradually. The photolysis rate constant also showed the same trend in Fig. 10. The removal rate of 2-CBA(30 mg/L) with TiO2 under the presence of phosphorus (0.5 mg/L) had fallen by 50% compared to that containing no phosphorus. Yoshiyuki came to the similar conclusion in the research about inactive MS2 with TiO2, in which the photocatalytic activity declined in presence of phosphorus (Yoshiyuki & Masahito, 2002). Especially the KH2PO4 added into the solution would dissociate into H+ and H2PO4−. The competitive adsorption between anion and 2-CBA reduced the efficiency of photocatalytic. Besides, H2PO4− had been used as scavenger for the hydroxyl radical, and it would also have inhibited the photodegradation of 2-CBA (Prieto-Rodriguez and Miralles-Cuevas, 2012).
Fig. 9.
The effect of H2P04− on the photodegradation of 2-CBA.
Fig. 10.
The kinetic analysis of H2P04- on the degradation of 2-CBA.
3.5. Effect of alcohol
In Fig. 11, it could be found that ethanol had a remarkable inhibition on the photodegradation of 2-CBA. With increase of the ethanol concentration, the inhibition rate raised to 51% after 4 h photodegradation, which showed that OH• played a critical fact in photodegradation process. Isopropanol was also introduced to confirm this result and then we get the same conclusion as before. It was noteworthy that under the same condition the photodegradation rate of 2-CBA was only 31% in presence of isopropanol.
Fig. 11.
The inhibition of alcohol on the photodegradation.
3.6. The photolysis pathway of 2-CBA
LC-MS analysis was used to evaluate the photodegradation reaction of 2-CBA. The samples showed the peaks with different retention time after separation by chromatographic column. Then the sample’s fragment ions were recorded according to the ratio of m/z under the comprehensive effect of electric field and magnetic field by mass spectrometry. Test result was shown in Fig. 12.
Fig. 12.
The scan of 2-CBA by LC/MS.
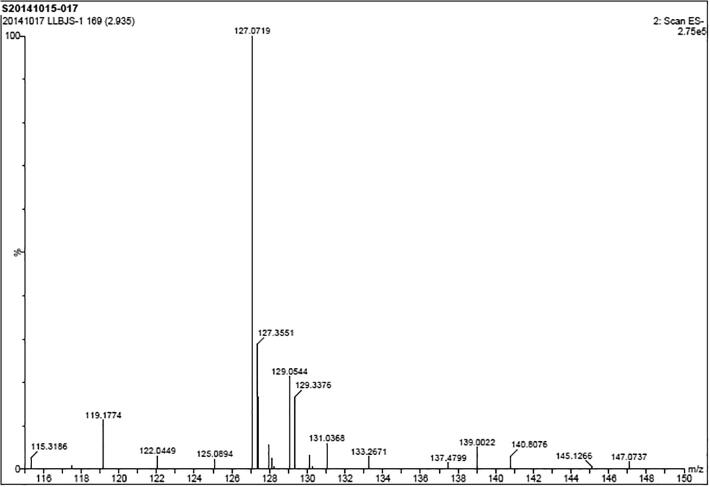
In Fig. 12, there are two obvious peaks appearing at 3.19 and 2.85 min. The peak appearing at 2.85 min was preliminarily identified as intermediate product by comparing with the full scan spectrum. The nuclear ratio of peak appearing at 3.19 min was 155/157 by mass spectrometry (Fig. 13), which was attributed to 2-CBA. Fig. 14 shows that the peak at 2.85 with m/z 127 tested under negative ion mode can be speculated as o-chlorophenol. This was due to that first the decarboxylation reaction of 2-CBA carried out easily and then the hydroxylation reaction of aromatic took place. It can satisfy the basic degradation pathway which we are concluded. The similar results had been bring out by Brett, Helene, Asma, Young, et al. (Faber et al., 2014, Ku et al., 1996, Vanderford et al., 2007, Wang et al., 2017, Wang et al., 2018, Zhang et al., 2019a, Zhang et al., 2019b).
Fig. 13.
The mass spectrometry of 2-CBA.
Fig. 14.
The mass spectrometry of intermediate material.
4. Conclusion
The optimal dosing quantity of TiO2 was 0.01 g for photocatalytic degradation 2-CBA(30 mg/L), and the removal rate reached as high as 82.9% after 4 h irradiation under UV light in pH3.5. Both NO3− and H2PO4− can make negative affect on the photodegradation of 2-CBA. It could be caused by the reduce of hydroxyl radicals or photogenerated hole which was produced by anionic in solution. By using alcohol as scavenger, hydroxyl radicals had been verified primarily that played an key role in the process of photocatalytic degradation towards organic matter with TiO2. By determining the intermediate product of 2-CBA, we found that photolysis pathways is first decarboxlation and then hydroxylation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Youth Fund of National Natural Science Foundation of China (51503148), Tianjin Research Program of Application Foundation and Advanced Technology (15JCQNJC09000), the Foundation for Outstanding Young Teachers in Tianjin Higher Education Institutions (TJYQ2013050012) and Youth Innovation Fund of Tianjin University of Science and Technology (Science & Reserch) (2014CXLG13).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abramovic Biljana F., Despotovic Vesna N., Šojic Daniela V. Photocatalytic degradation of the herbicide clomazone in natural water using TiO2: kinetics, mechanism, and toxicity of degradation products. Chemosphere. 2013;93:166–171. doi: 10.1016/j.chemosphere.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Antoine P., Didier R., Jean V.W. Influence of pH and chloride anion on the photocatalytic degradation of organic compounds Part I. Effect on the benzamide and para-hydroxybenzoic acid in TiO2 aqueous solution. Appl. Catal. B. 2001;35:117–124. [Google Scholar]
- Barndõk H., Hermosilla D., Cortijo L. Assessing the effect of inorganic anions on TiO2-photocatalysis and ozone oxidation treatment efficiencies. J. Adv. Oxid. Technol. 2012;15(8):125–132. [Google Scholar]
- Blackledge C.A., Partridge E.A., Wilson I.D. The metabolism and excretion of 14C 2-and 4-chlorobenzoic acids in the rat. J. Pharm. Biomed. Anal. 2000;22(6):1023–1028. doi: 10.1016/s0731-7085(00)00295-8. [DOI] [PubMed] [Google Scholar]
- Cassani S., Kovarich S., Papa E. Daphnia and fish toxicity of (benzo) triazoles: validated QSAR models, and interspecies quantitative activity–activity modeling. J. Hazard. Mater. 2013;258:50–60. doi: 10.1016/j.jhazmat.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Faber H., Lutze H., Lareo P.L. Liquid chromatography/mass spectrometry to study oxidative degradation of environmentally relevant pharmaceuticals by electrochemistry and ozonation. J. Chromatogr. A. 2014;1343:152–159. doi: 10.1016/j.chroma.2014.03.081. [DOI] [PubMed] [Google Scholar]
- Garnier J., Némery J., Billen G. Nutrient dynamics and control of eutrophication in the Marne River system: modelling the role of exchangeable phosphorus. J. Hydrol. 2005;304(1):397–412. [Google Scholar]
- Gichner T., Lovecka P., Vrchotova B. Genomic damage induced in tobacco plants by chlorobenzoic acids—metabolic products of polychlorinated biphenyls. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008;657(2):140–145. doi: 10.1016/j.mrgentox.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Haque M.M., Muneer M. Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide. J. Hazard. Mater. 2007;145(1):51–57. doi: 10.1016/j.jhazmat.2006.10.086. [DOI] [PubMed] [Google Scholar]
- Ku Y., Leu R.M., Lee K.C. Decomposition of 2-chlorophenol in aqueous solution by UV irradiation with the presence of titanium dioxide. Water Res. 1996;30(11):2569–2578**. [Google Scholar]
- Lenhart H.J., Mills D.K., Baretta-Bekker H. Predicting the consequences of nutrient reduction on the eutrophication status of the North Sea. J. Mar. Syst. 2010;81(1):148–170. [Google Scholar]
- Li X., Zhang Q., Tang L. Catalytic ozonation of p-chlorobenzoic acid by activated carbon and nickel supported activated carbon prepared from petroleum coke. J. Hazard. Mater. 2009;163(1):115–120. doi: 10.1016/j.jhazmat.2008.06.068. [DOI] [PubMed] [Google Scholar]
- Liang H., Li X., Yang Y. Effects of dissolved oxygen, pH, and anions on the 2, 3-dichlorophenol degradation by photocatalytic reaction with anodic TiO2 nanotube films. Chemosphere. 2008;73(5):805–812. doi: 10.1016/j.chemosphere.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Lin Hank Hui-Hsiang, Lin Angela Yu-Chen. Photocatalytic oxidation of 5-fluorouracil and cyclophosphamide via UV/TiO2 in an aqueous environment. Water Res. 2014;48:559–568. doi: 10.1016/j.watres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Liu H., Yin B. Annual cycle of carbon, nitrogen and phosphorus in the Bohai Sea: a model study. Cont. Shelf Res. 2007;27(10):1399–1407. [Google Scholar]
- Liu H., Yin B. Numerical investigation of nutrient limitations in the Bohai Sea. Mar. Environ. Res. 2010;70(3):308–317. doi: 10.1016/j.marenvres.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Maciuca A., Batiot-Dupeyrat C., Tatibouët J.M. Synergetic effect by coupling photocatalysis with plasma for low VOCs concentration removal from air. Appl. Catal. B. 2012;125:432–438. [Google Scholar]
- Mhemdi A., Oturan M.A., Oturan N. Electrochemical advanced oxidation of 2-chlorobenzoic acid using BDD or Pt anode and carbon felt cathode. J. Electroanal. Chem. 2013;709:111–117. [Google Scholar]
- Muzikář M., Křesinová Z., Svobodová K. Biodegradation of chlorobenzoic acids by ligninolytic fungi. J. Hazard. Mater. 2011;196:386–394. doi: 10.1016/j.jhazmat.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Nakata K., Fujishima A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C. 2012;13(3):169–189. [Google Scholar]
- Neppolian B., Park J.S., Choi H. Effect of Fenton-like oxidation on enhanced oxidative degradation of para-chlorobenzoic acid by ultrasonic irradiation. Ultrason. Sonochem. 2004;11(5):273–279. doi: 10.1016/j.ultsonch.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Pelaez M., Nolan N.T., Pillai S.C. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B. 2012;125:331–349. [Google Scholar]
- Prieto-Rodriguez L., Miralles-Cuevas S. Optimization of mild solar TiO2 photocatalysis as a tertiary treatment for municipal wastewater treatment plant effluents. Appl. Catal. B. 2012;128:119–125. [Google Scholar]
- Rincon A.G., Pulgarin C. Effect of pH, inorganic ions, organic matter and H2O2 on E-coli K12 photocatalytic inactivation by TiO2-Implications in solar water disinfection. Appl. Catal. B. 2004;51(4):283–302. [Google Scholar]
- Santiago D.E., Araña J., González-Díaz O. Effect of inorganic ions on the photocatalytic treatment of agro-industrial wastewaters containing imazalil. Appl. Catal. B. 2014;156:284–292. [Google Scholar]
- Vanderford B.J., Rosario-Ortiz F.L., Snyder S.A. Analysis of p-chlorobenzoic acid in water by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2007;1164(1):219–223. doi: 10.1016/j.chroma.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Wang K., Li L., Xue W., Zhou S., Lan Y., Zhang H., Sui Z. Electrodeposition synthesis of PANI/MnO2/Graphene composite materials and its electrochemical performance. Int. J. Electrochem. Sci. 2017;12:8306–8314. [Google Scholar]
- Wang Y., Lu K., Feng C. Influence of inorganic anions and organic additives on photocatalytic degradation of methyl orange with supported polyoxometalates as photocatalyst. J. Rare Earths. 2013;31(4):360–365. [Google Scholar]
- Wang K., Zhou S., Zhou Y., Ren J., Li L., Lan Y. Synthesis of porous carbon by activation method and its electrochemical performance. Int. J. Electrochem. Sci. 2018;13:10766–10773. [Google Scholar]
- Yoshiyuki K., Masahito T. Photocatalytic inactivation rate of phage MS2 in titanium dioxide suspensions containing various ionic species. Biotechnol. Lett. 2002;24(6):459–462. [Google Scholar]
- Yu J.C., Ho W., Yu J. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 2005;39(4):1175–1179. doi: 10.1021/es035374h. [DOI] [PubMed] [Google Scholar]
- Zhang L., Chen J., Zhang L., He H., Wang Y., Yonghui L. Preparation of soybean oil factory sludge catalyst and its application in selective catalytic oxidation denitration process. J. Cleaner Prod. 2019;225:220–226. [Google Scholar]
- Zhang L., Jia Y., Zhang L., He H., Yang C., Luo M., Miao L. Preparation of soybean oil factory sludge catalyst by plasma and the kinetics of selective catalytic oxidation denitrification reaction. J. Cleaner Prod. 2019;217:317–323. [Google Scholar]
- Zhu Z.Y., Wu Y., Zhang J. Reconstruction of anthropogenic eutrophication in the region off the Changjiang Estuary and central Yellow Sea: From decades to centuries. Cont. Shelf Res. 2014;72:152–162. [Google Scholar]