Abstract
OBJECTIVE
The effects of preventive interventions on cardiovascular autonomic neuropathy (CAN) remain unclear. We examined the effect of intensively treating traditional risk factors for CAN, including hyperglycemia, hypertension, and dyslipidemia, in individuals with type 2 diabetes (T2D) and high cardiovascular risk participating in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.
RESEARCH DESIGN AND METHODS
CAN was defined as heart rate variability indices below the fifth percentile of the normal distribution. Of 10,251 ACCORD participants, 71% (n = 7,275) had a CAN evaluation at study entry and at least once after randomization. The effects of intensive interventions on CAN were analyzed among these subjects through generalized linear mixed models.
RESULTS
As compared with standard intervention, intensive glucose treatment reduced CAN risk by 16% (odds ratio [OR] 0.84, 95% CI 0.75–0.94, P = 0.003)—an effect driven by individuals without cardiovascular disease (CVD) at baseline (OR 0.73, 95% CI 0.63–0.85, P < 0.0001) rather than those with CVD (OR 1.10, 95% CI 0.91–1.34, P = 0.34) (Pinteraction = 0.001). Intensive blood pressure (BP) intervention decreased CAN risk by 25% (OR 0.75, 95% CI 0.63–0.89, P = 0.001), especially in patients ≥65 years old (OR 0.66, 95% CI 0.49–0.88, P = 0.005) (Pinteraction = 0.05). Fenofibrate did not have a significant effect on CAN (OR 0.91, 95% CI 0.78–1.07, P = 0.26).
CONCLUSIONS
These data confirm a beneficial effect of intensive glycemic therapy and demonstrate, for the first time, a similar benefit of intensive BP control on CAN in T2D. A negative CVD history identifies T2D patients who especially benefit from intensive glycemic control for CAN prevention.
Introduction
Cardiovascular autonomic neuropathy (CAN) is a common and severe complication of diabetes that independently predicts cardiovascular disease (CVD) morbidity and mortality among people with this condition (1,2). Defined as the impairment of autonomic control of the cardiovascular system (1), CAN prevalence rates vary based on the populations studied as well as disease duration and burden, with rates as high as 50–90% among people with long-standing diabetes (1). In addition, evidence of various degrees of cardiovascular autonomic dysfunction has been described in several cohorts of patients with prediabetes/metabolic syndrome (3–5) and in youth (6). At its initial stages, CAN is asymptomatic and thus easily overlooked, as changes in heart rate variability (HRV) may be the only findings (1). At more advanced stages, CAN manifests itself as resting tachycardia, exercise intolerance, orthostatic hypotension, syncope, and silent myocardial infarction and ischemia (1). Electrocardiogram (ECG)-derived indices of HRV are commonly used for an early diagnosis of CAN, as indicators of the underlying pathogenesis of CAN (4,7). These indices of HRV reflect the imbalance between cardiac parasympathetic and sympathetic tone and have important prognostic implications (4,7,8).
Known risk factors for CAN include duration of diabetes, degree of hyperglycemia, obesity, dyslipidemia, hypertension, and smoking (9). The Steno-2 (Intensified Multifactorial Intervention in Patients With Type 2 Diabetes and Microalbuminuria) trial demonstrated a 63% reduction in the rate of progression to CAN in individuals with type 2 diabetes (T2D) via intensive multifactorial interventions targeting hyperglycemia, hypertension, dyslipidemia, and lifestyle (10). On this basis, current clinical practice guidelines recommend a multifactorial approach to prevent CAN including all of the above interventions (1). However, these treatments have evolved since Steno-2 as more recent trials of glucose, blood pressure (BP), and lipid control have used more stringent targets (11–13). Moreover, the individual effects of any of these interventions on CAN in patients with T2D have not been systematically evaluated, as the majority of the prior trials did not measure or analyze CAN (1).
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial investigated the effects of intensive glycemic, BP, and lipid interventions on CVD events in participants with T2D and high cardiovascular risk (14). The trial had a factorial design allowing the examination of each intervention independent from the other two. After 3.5 years of follow-up, ACCORD reported a beneficial effect of an intensive glucose-lowering strategy on nonfatal cardiovascular events, but this was accompanied by a paradoxical increase in mortality (15). The presence of CAN at baseline was associated with a 1.5- to 2.1-fold increase in mortality depending on its severity (16). This finding did not explain the excess mortality in the intensive glycemic treatment arm but established CAN as an independent predictor of mortality in T2D.
The goal of this study was to assess the effects of the glycemia, BP, and lipid interventions on CAN during ACCORD.
Research Design and Methods
ACCORD
The study design of ACCORD has previously been described (14). Briefly, the trial aimed to investigate whether cardiovascular event rates could be reduced by intensive targeting of three important CVD risks: hyperglycemia, hypertension, and dyslipidemia. For this purpose, 10,251 ACCORD participants with T2D and high cardiovascular risk were randomized in a 1:1 ratio to receive intensive (targeting HbA1c <6.0% [42 mmol/mol]) or standard (targeting HbA1c 7–7.9% [53–64 mmol/mol]) glycemia-lowering therapy at 77 clinical sites across the U.S. and Canada (14). With application of a 2 × 2 factorial design, 4,733 participants were also randomly assigned to intensive BP therapy (targeting systolic BP [SBP] <120 mmHg) or standard therapy (targeting SBP <140 mmHg), and 5,518 participants who were being treated with open-label simvastatin were randomly assigned to either masked fenofibrate or placebo. Recruitment and randomization occurred in two phases: 1,174 participants from January 2001 to June 2001, ACCORD Vanguard Phase, and 9,077 participants from February 2003 to October 2005, ACCORD main study phase. Due to the higher mortality in the intensive glycemic arm adjudicated during the study, participants in the intensive glycemic arm were switched to the standard glycemic arm after a mean treatment period of 3.7 years (calendar time 5 February 2008). ACCORD BP and ACCORD Lipid subtrials were maintained as prespecified until the planned end of the trial, i.e., for an additional 17 months. ACCORD ended in June 2009. The primary outcome of ACCORD was adjudicated as the first occurrence of a major cardiovascular event, which was defined as the composite of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death (14). Other outcomes included microvascular complications, hypoglycemia, cognitive dysfunction, and quality of life. Data on these clinical outcomes were continuously collected and adjudicated as per prespecified algorithms throughout the study.
The trial was conducted in accordance with the Declaration of Helsinki, the International Ethical Guidelines for Health-Related Research Involving Humans of the Council for International Organizations of Medical Sciences in collaboration with the World Health Organization, and the Good Clinical Practice guidelines of the International Conference on Harmonization. The protocol was reviewed and approved by all local institutional review boards, and all participants provided written informed consent.
Evaluation and Outcome Definition of CAN
Measures of CAN were derived from 12-lead digitized ECGs, recorded over 10 consecutive seconds with the patient resting supine after an overnight fast as previously described (16). ECG tracings were obtained at baseline, every 2 years, and at the last trial visit. If ECGs were acquired at all of these time points, participants from the ACCORD Vanguard Phase had a maximum of five visits with CAN data (baseline and 2, 4, 6, and 7 years after randomization), whereas participants from the ACCORD main study phase had a maximum of four visits with CAN data (baseline and 2, 4, and 5 years after randomization). Digitalization, quality control, and inclusion/exclusion criteria of ECG in ACCORD have previously been reported (16).
In the report by the ACCORD Study Group on CAN as predictor of mortality, CAN was defined as the combination of 1) an SD of all normal-to-normal R-Rs (SDNN) in the lowest quartile and 2) a QT index in the highest quartile of the ACCORD population (SDNN <7.815 ms and QT index >104.32%) (16), given the different scope of those analyses. However, since the Multi-Ethnic Study of Atherosclerosis (MESA) recently published new cutoff data for indices of HRV derived from a large sample of healthy participants (17), CAN was defined in the present analyses as SDNN and root mean square of successive differences between normal-to-normal R-Rs (rMSSD) both being below the fifth percentile of the general population distributions, i.e., SDNN <8.2 ms and rMSSD <8.0 ms.
Statistical Analysis
All statistical analyses were conducted in SAS, version 9.4 (SAS Institute, Cary, NC). An intention-to-treat approach was applied.
For descriptive purposes, normally distributed continuous variables are presented as means ± SD and analyzed by independent t tests for difference in means between groups. Non–normally distributed continuous variables are presented as medians and interquartile ranges (IQRs) and analyzed by t tests after log transformation. Categorical variables are presented as counts (percentages) and analyzed by χ2 tests to examine differences among groups.
The effect of treatments on CAN prevalence during follow-up was analyzed by means of generalized linear mixed models with a binary response distribution and logit link function using the PROC GLIMMIX procedure of SAS. For this analysis, we defined CAN as present (1) or absent (0) at each time point during follow-up based on the corresponding CAN assessment, including subject identifier as a random effect to account for the repeated CAN observations within each subject. We fitted two basic models: one (minimally adjusted model) that included only a linear time effect, CVD history, baseline CAN status, clinical center network, and treatment assignments as independent variables and another (fully adjusted model) that also controlled for baseline covariates, namely, age, sex, race, diabetes duration, HbA1c, BMI, height, alcohol, cigarettes, SBP and DBP, LDL cholesterol, triglycerides, HDL cholesterol, urinary albumin-to-creatinine ratio (UACR), and use of thiazolidinediones, insulin, β-blockers, ACE inhibitors (ACEI)/angiotensin II receptor blocker (ARB), and statins. For both minimally and fully adjusted models, we included four indicator variables to reflect treatment assignments: one reflecting randomization to intensive versus standard glycemia, another representing allocation to the ACCORD BP versus ACCORD Lipid subtrials, a third reflecting randomization to intensive or standard BP goals (and set to 0 for ACCORD Lipid participants), and a fourth reflecting randomization to fenofibrate or placebo in ACCORD Lipid (and set to 0 for ACCORD BP participants). The effects of treatments were expressed as odds ratios (ORs) representing the odds of being positive for CAN during the postrandomization period among participants in the intensive treatment arms as compared with the odds among participants in the standard treatment groups. The possible influence of the concomitant use of β-blockers and ACEI/ARB, as well as the development of CVD during the trial, was evaluated by further adjustment for the generalized linear mixed model for a time-dependent index of β-blocker and ACEI/ARB use and CVD events. Finally, the effect of death as a competing risk was evaluated through sensitivity analyses estimating the proportion of deaths in the intensive glycemic arm that had to be due to CAN for the effect of intensive glycemic intervention to lose significance in a competing risk analysis. Logistic regression models including these same covariates as in the mixed models were used to compare CAN prevalence between intervention arms at each visit.
The effects of ACCORD interventions on SDNN and rMSSD considered as continuous outcomes were investigated by means of linear mixed regression models, with application of unstructured covariance and random effects of patients and inclusion of the same covariates used for the analysis of the dichotomous CAN outcome, with baseline CAN status being replaced by baseline SDNN or rMSSD.
To test for heterogeneity in the effect of glycemic control, we conducted stratified analyses using the clinical subgroups prespecified by ACCORD investigators to examine the effects of interventions on cardiovascular outcomes (14). The significance of differences in effect between clinical subgroups was evaluated by addition of appropriate interaction terms to the mixed models. The interaction between intensive glycemic intervention and the other two interventions investigated in the trial (BP control and dyslipidemia management) was similarly evaluated.
Data and Resource Availability
The ACCORD database is available upon request from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository (https://biolincc.nhlbi.nih.gov/studies/accord/).
Results
Study Cohort
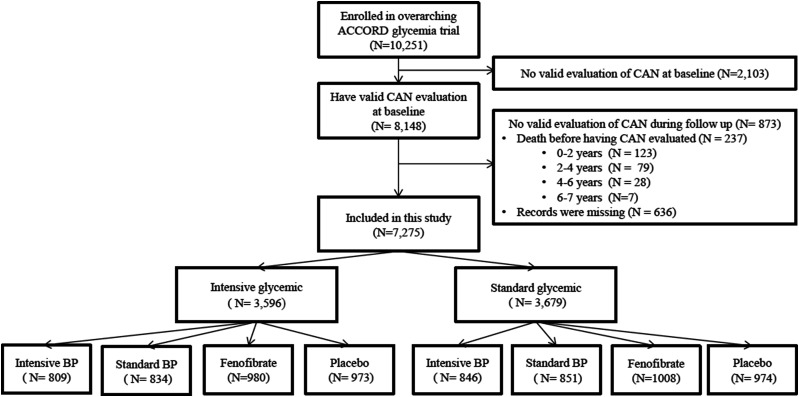
This post hoc analysis of ACCORD included 7,275 ACCORD participants (71% of the total) with valid CAN data at study entry and at least once in the postrandomization period (Fig. 1). As compared with the rest of the ACCORD cohort, the participants included in this study were slightly younger and had slightly shorter duration of diabetes, and the group included a higher proportion of women. They also had slightly higher total cholesterol, triglycerides, estimated glomerular filtration rate, and diastolic BP [DBP]) and lower prevalence of self-reported retinopathy and CVD history, as well as lower UACR, and were less likely to use insulin or smoke (Supplementary Table 1). Among the participants included in the study, there were no significant differences between treatment arms in the number of CAN evaluations during follow-up (Supplementary Table 2).
Figure 1.
Flow diagram of the study population included in the analysis. Intensive and standard glycemic and BP refer to intensive and standard glycemic and BP interventions.
Effect of Intensive Versus Standard Glycemia Intervention on CAN
Of the 7,275 participants included in this study, 3,596 were assigned to the intensive and 3,679 to the standard arm of the glycemia trial (Fig. 1). As shown in Table 1, baseline characteristics were balanced between the two treatment groups, except for the intensive glycemia group including more current smokers and fewer insulin users. The median HbA1c at baseline was 8.1% in both groups. In the standard glycemia group, the median HbA1c decreased to 7.5% (IQR 7.0–8.2) during treatment. In the intensive glycemia group, HbA1c decreased to 6.4% (IQR 6.2–7.2), remained at this level until the intensive glycemia strategy was discontinued after a median follow-up of 47 months (IQR 36–56) (Supplementary Fig. 1), and then progressively rose to 7.2% (IQR 6.6–7.9) at the final visit.
Table 1.
Baseline characteristics by ACCORD trial assignment groups
| Glycemia trial‡ | ACCORD BP‡ | ACCORD Lipid‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensive | Standard | P | Intensive | Standard | P | Fenofibrate | Placebo | P | |
| (N = 3,596) | (N = 3,679) | (N = 1,655) | (N = 1,685) | (N = 1,988) | (N = 1,947) | ||||
| Female | 1,438 (40.0) | 1,474 (40.1) | 0.95 | 787 (47.6) | 826 (49) | 0.40 | 665 (33.5) | 634 (32.6) | 0.55 |
| Age (years) | 62.3 ± 6.4 | 62.4 ± 6.5 | 0.30 | 62.4 ± 6.4 | 62.3 ± 6.7 | 0.78 | 62.3 ± 6.3 | 62.4 ± 6.5 | 0.73 |
| DM duration (years) | 10.6 ± 7.5 | 10.8 ± 7.5 | 0.33 | 10.8 ± 7.7 | 10.9 ± 7.6 | 0.84 | 10.6 ± 7.3 | 10.5 ± 7.4 | 0.83 |
| BMI (kg/m2) | 32.2 ± 5.4 | 32.2 ± 5.3 | 0.83 | 32.1 ± 5.6 | 32.0 ± 5.3 | 0.64 | 32.3 ± 5.3 | 32.4 ± 5.4 | 0.62 |
| Waist (cm) | 106.5 ± 13.7 | 106.5 ± 13.4 | 0.96 | 105.9 ± 14.1 | 105.0 ± 13.1 | 0.05 | 107.3 ± 13.3 | 107.5 ± 13.6 | 0.54 |
| Height (cm) | 170.0 ± 9.8 | 170.0 ± 9.8 | 0.50 | 169.0 ± 10.1 | 168.8 ± 9.7 | 0.48 | 170.1 ± 9.6 | 170.1 ± 9.8 | 0.30 |
| HbA1c (%)* | 8.1 (7.5–8.8) | 8.1 (7.6–8.9) | 0.13 | 8.2 (7.6–8.9) | 8.1 (7.6–8.8) | 0.09 | 8.1 (7.5–8.8) | 8.0 (7.5–8.8) | 0.10 |
| Fasting glucose (mg/dL) | 174.0 ± 52.5 | 176.0 ± 53.3 | 0.10 | 175.1 ± 53.9 | 173.4 ± 53.2 | 0.35 | 176.0 ± 51.6 | 175.2 ± 53.1 | 0.66 |
| SBP (mmHg) | 135.8 ± 16.2 | 136.1 ± 16.4 | 0.54 | 138.9 ± 15.4 | 138.8 ± 14.7 | 0.93 | 133.4 ± 16.8 | 133.5 ± 17 | 0.78 |
| DBP (mmHg) | 75.0 ± 10 | 74.8 ± 10.1 | 0.61 | 76.0 ± 10.0 | 75.9 ± 9.5 | 0.76 | 74.0 ± 10.1 | 74.0 ± 10.3 | 0.66 |
| LDL (mg/dL) | 104.8 ± 32.9 | 104.8 ± 33.1 | 0.95 | 110.2 ± 36.1 | 109.1 ± 34.5 | 0.37 | 100.1 ± 29.9 | 101.2 ± 30.8 | 0.28 |
| HDL (mg/dL) | 41.9 ± 11.2 | 41.7 ± 10.8 | 0.40 | 46 ± 12.6 | 46 ± 12.9 | 0.96 | 38.2 ± 7.7 | 38.3 ± 7.6 | 0.73 |
| Women | 47.0 ± 12.1 | 46.4 ± 11.7 | 0.19 | 50.9 ± 12.8 | 50.9 ± 13.2 | 0.93 | 41.4 ± 7.7 | 41.6 ± 7.6 | 0.64 |
| Men | 38.5 ± 9.0 | 38.5 ± 8.9 | 0.96 | 41.5 ± 10.6 | 41.3 ± 10.6 | 0.71 | 36.6 ± 7.2 | 36.7 ± 7.1 | 0.75 |
| Total cholesterol (mg/dL) | 183.5 ± 40.0 | 183.6 ± 40.3 | 0.93 | 193.2 ± 42.8 | 192 ± 41.3 | 0.41 | 175.2 ± 35.9 | 176.5 ± 37.5 | 0.30 |
| Triglycerides (mg/dL)* | 159 (107.5–232) | 156 (109–231) | 0.70 | 150 (100.5–229) | 150 (99–229) | 0.98 | 165 (116–235) | 163 (114–233) | 0.63 |
| eGFR (mL/min/1.73 m2) | 90.8 ± 22.7 | 90.7 ± 22.3 | 0.87 | 91.5 ± 23.6 | 90.8 ± 22.8 | 0.38 | 90.9 ± 21.9 | 90 ± 21.8 | 0.20 |
| UACR (mg/mmol)* | 1.3 (0.7–4.1) | 1.3 (0.7–4.3) | 0.68 | 1.4 ((0.7–4.1) | 1.4 (0.7–4.4) | 0.56 | 1.3 (0.7–4.3) | 1.3 (0.7–3.9) | 0.63 |
| Previous CV event† | 1,203 (33.5) | 1,213 (33.0) | 0.66 | 522 (31.5) | 546 (32.4) | 0.59 | 674 (33.9) | 674 (34.6) | 0.64 |
| Report of retinopathy | 349 (11.0) | 368 (11.3) | 0.65 | 161 (10.7) | 166 (11.1) | 0.74 | 189 (11) | 201 (11.8) | 0.46 |
| Current smoker | 443 (12.3) | 396 (10.8) | 0.04 | 187 (11.3) | 188 (11.2) | 0.90 | 249 (12.5) | 215 (11) | 0.15 |
| Previous smoker | 1,612 (51.6) | 1,627 (50.1) | 0.22 | 723 (49.7) | 708 (47.8) | 0.31 | 932 (54) | 876 (51.3) | 0.12 |
| Insulin therapy | 1,179 (32.9) | 1,314 (35.8) | 0.01 | 593 (36) | 616 (36.7) | 0.68 | 654 (33) | 630 (32.5) | 0.71 |
Except where noted, data are means ± SD for continuous variables and counts (%) for categorical data. CV, cardiovascular; DM, diabetes; eGFR, estimated glomerular filtration rate.
Medians (IQR).
Prior cardiovascular event: In ACCORD, this includes secondary prevention status or history of myocardial infarction, stroke, angina, and/or ischemic changes (ECG) on graded exercise tolerance test or positive imaging, coronary revascularization procedures, or other revascularization procedures at baseline.
The glycemia trial was the overarching trial in ACCORD. All the participants in the glycemia trial were randomized to the ACCORD BP or ACCORD Lipid subtrials according to a two-by-two-by-two factorial design.
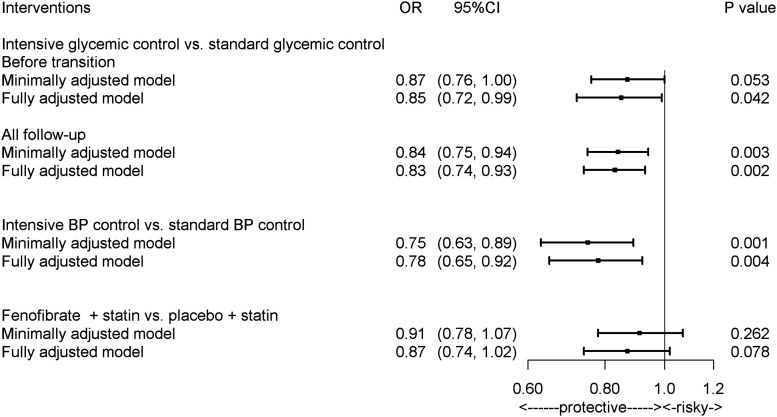
The prevalence of CAN at study entry was similar in the two treatment arms (19.9% in the intensive arm vs. 19.0% in the standard arm, P = 0.30). However, in the postrandomization period, the odds of CAN were on average lower in the intensive than in the standard arm (OR 0.84, 95% CI 0.75–0.94, P = 0.003) (Fig. 2). The largest differences between treatments were observed at 4 years (OR 0.80, 95% CI 0.70–0.91, P = 0.0009) and 6 years (OR 0.78, 95% CI 0.63–0.97, P = 0.03) (Supplementary Fig. 2). A similar difference in CAN prevalence was found between treatment arms in an analysis limited to the time interval between randomization and transition from intensive to standard glycemia targets (OR 0.87, 95% CI 0.76–1.00, P = 0.05) (Fig. 2). Consistent with these findings, SDNN and rMSSD, analyzed as continuous variables, were higher in the intensive than in the standard glycemic arm, although statistical significance was reached only for rMSSD (Supplementary Fig. 3).
Figure 2.
Effects of interventions in ACCORD on CAN. The primary model was adjusted by fixed effects including trial assignments, seven clinical center networks, time after randomization, prior CVD events, and baseline CAN status. The full model was adjusted by additional baseline characteristics as fixed effects, namely, age, sex, diabetes duration, HbA1c, BMI, height, alcohol, cigarettes, SBP and DBP, LDL cholesterol, triglycerides, HDL cholesterol, UACR, and use of thiazolidinediones, insulin, β-blockers, ACEIs/ARB, and statins. Both models included participants as random effects.
The protective effect of the intensive glycemia intervention on CAN was not affected by adjustment for the known baseline CAN predictors described in research design and methods (OR 0.83, 95% CI 0.74–0.93, P = 0.002, in the analysis until the end of the trial, and OR 0.85, 95% CI 0.72–0.99, P = 0.04, in the analysis until transition to standard treatment) (Fig. 2), for the concomitant use of β-blockers or ACEI/ARB during the trial (OR 0.82, 95% CI 0.73–0.93, P = 0.001, until the end of trial, and OR 0.86, 95% CI 0.75–0.99, P = 0.04, until transition to standard treatment), or for the occurrence of CVD events (Supplementary Table 3). This effect remained robust in sensitivity analyses assessing the potential impact of death as a competing risk. Of 8,148 participants who had CAN evaluated at baseline, 511 died: 237 before they could be evaluated for CAN during follow-up and 274 after CAN had been evaluated at least once. The observed effect of the intensive glycemia intervention lost significance in the competing risk analysis only if CAN was assumed to be the cause of >30% of deaths in the intensive arm and of none of the deaths in the standard arm—a very unlikely occurrence.
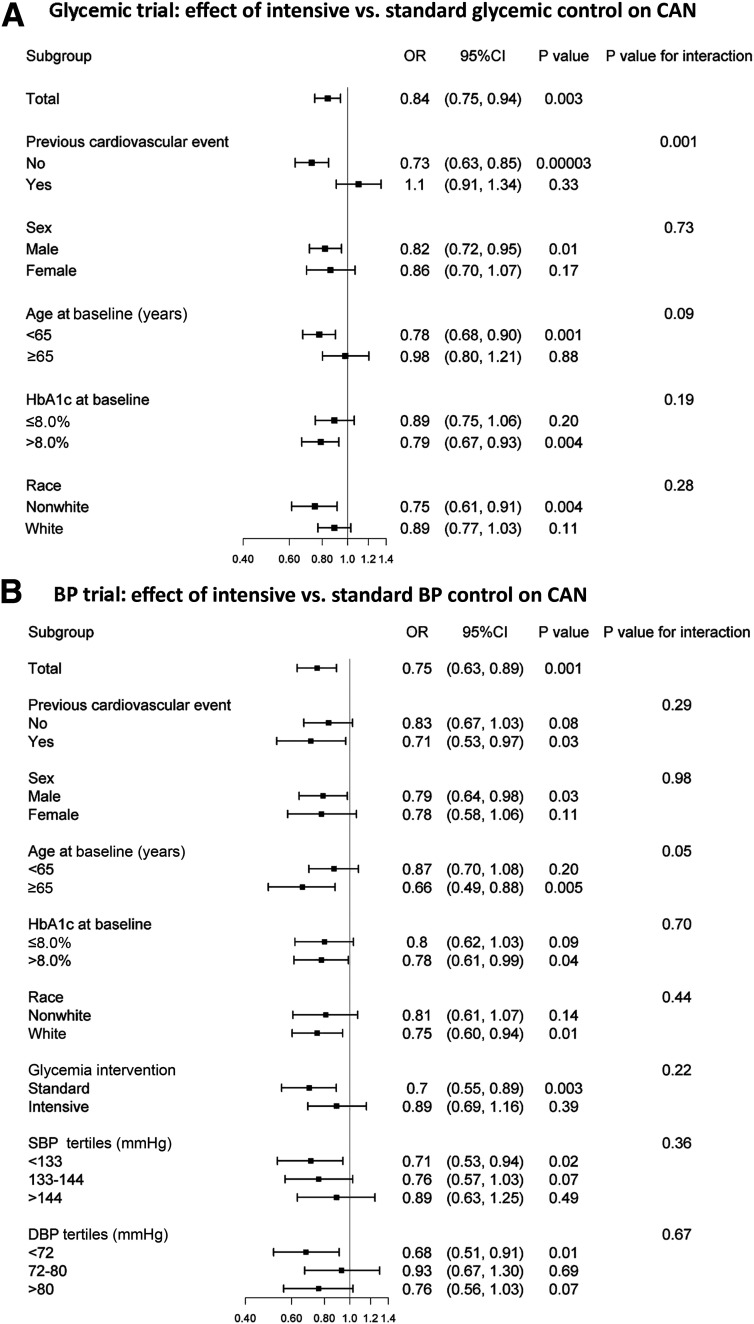
In subgroup analyses based on the stratifying variables prespecified for the ACCORD primary outcome (Fig. 3A), the protective effect of the intensive glycemia intervention on CAN was found among participants without a history of CVD events at study entry (OR 0.73, 95% CI 0.63–0.85, P < 0.0001), but not among those with a positive CVD history (OR 1.10, 95% CI 0.91–1.34, P = 0.33), resulting in a significant interaction between the two exposures (P = 0.001). No other stratifying variable displayed a positive or negative interaction with intensive glycemic intervention.
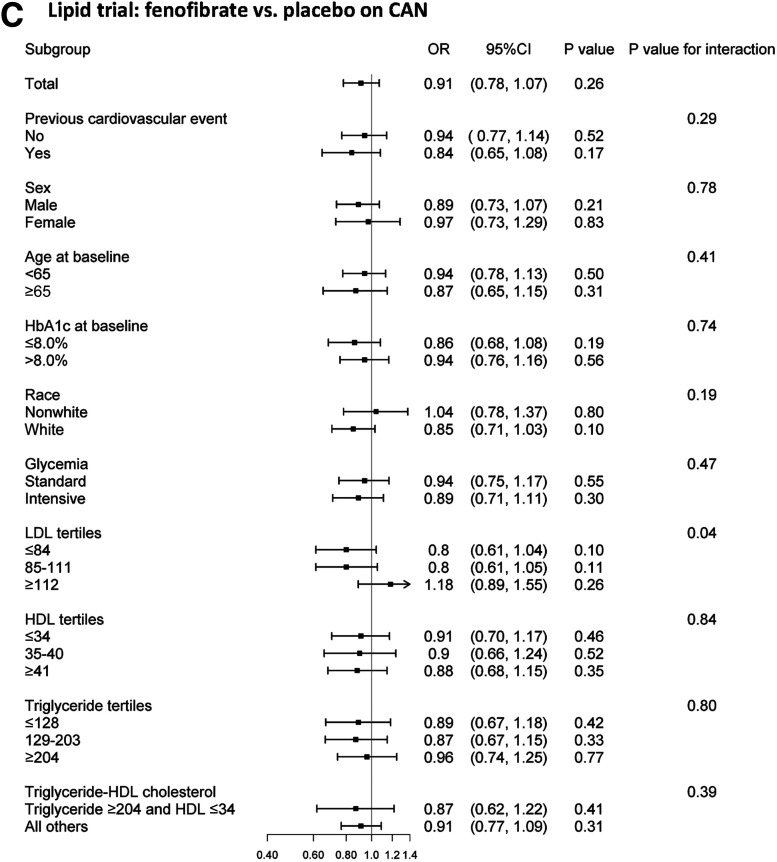
Figure 3.
Effects of ACCORD interventions on CAN risk in the subgroups prespecified for the analysis of the ACCORD primary outcome.
Effect of Intensive Versus Standard BP Intervention on CAN
Of the 7,275 participants included in this analysis, 3,340 were also enrolled in the ACCORD BP subtrial: 1,655 of them assigned to receive intensive BP control and 1,685 assigned to receive standard BP control (Fig. 1). Baseline characteristics were balanced between the two treatment groups (Table 1). The mean SBP and DBP at baseline were 138.9 and 76.0 mmHg in the intensive BP treatment group and 138.8 and 75.9 mmHg in the standard BP treatment group, respectively. During the trial, SBP and DBP decreased to average values of 120.9 and 65.0 mmHg in the intensive treatment group as compared with average values of 133.7 and 70.2 mmHg in the standard treatment group (P < 0.0001 for both SBP and DBP).
The prevalence of CAN at study entry was 18.4% in the intensive BP arm and 18.9% in the standard arm (P = 0.71). During the entire trial, incidence of CAN was lower in the intensive than in the standard BP arm (OR 0.75, 95% CI 0.63–0.89, P = 0.001) (Fig. 2 and Supplementary Fig. 2B). The largest difference between treatment arms was observed at 2 years (OR 0.76, 95% CI 0.63–0.92, P = 0.005). Adjustment for the time-dependent occurrence of cardiovascular events during follow-up did not change the magnitude of this effect (Supplementary Table 3). When HRV indices were analyzed as continuous variables, intensive BP control significantly increased rMSSD by 6% and nonsignificantly increased SDNN by 3% (Supplementary Fig. 3). These effects were similar in the fully adjusted model. The effect of the BP intervention on CAN was especially visible among participants with age ≥65 years (OR 0.66, 95% CI 0.49–0.88, P = 0.005, P for BP intervention by age interaction = 0.05) (Fig. 3).
Effect of Fenofibrate Versus Placebo on CAN
Of the 7,275 participants included in this analysis, 3,935 were also enrolled in the ACCORD Lipid subtrial: 1,988 were assigned to receive fenofibrate and 1,947 to receive placebo in addition to statins to control dyslipidemia (Fig. 1). The two treatment groups had similar baseline characteristics (Table 1). The mean LDL cholesterol dropped from 100.2 mg/dL to 82.3 mg/dL in the fenofibrate group and from 101.2 mg/dL to 80.1 mg/dL in the placebo group. The mean HDL cholesterol levels increased from 38.2 mg/dL at baseline to 41.4 mg/dL in the fenofibrate group and from 38.3 mg/dL to 40.5 mg/dL in the placebo group. Median plasma triglyceride levels decreased from 165 mg/dL to 122 mg/dL in the fenofibrate group and from 163 mg/dL to 144 mg/dL in the placebo group.
At study entry, 19.8% of participants in the fenofibrate group had evidence of CAN as compared with 20.5% in the placebo group (P = 0.60). No significant differences in the odds of CAN were observed between treatment groups during the treatment period (OR 0.91, 95% CI 0.77–1.06, P = 0.26, in the unadjusted analysis, and OR 0.87, 95% CI 0.74–1.02, P = 0.08, in the adjusted analysis) (Fig. 2 and Supplementary Fig. 2C). Adjustment by concurrent cardiovascular events did not affect such differences (Supplementary Table 3). However, when analyzed as continuous variables, both SDNN and rMSSD were significantly higher throughout the trial in the fenofibrate group in both unadjusted (β = 1.04, 95% CI 1.01–1.08, P = 0.03, and β = 1.06, 95% CI 1.02–1.10, P = 0.003, respectively) and fully adjusted (β = 1.03, 95% CI 1.00–1.07, P = 0.04, and β = 1.05, 95% CI 1.02–1.09, P = 0.002, respectively) analyses (Supplementary Fig. 3). These results were not affected by further adjustment for the concomitant use of β-blockers or ACEI/ARB during the trial (β = 1.04, 95% CI 1.00–1.08, P = 0.03, and β = 1.06, 95% CI 1.02–1.10, P = 0.003, respectively).
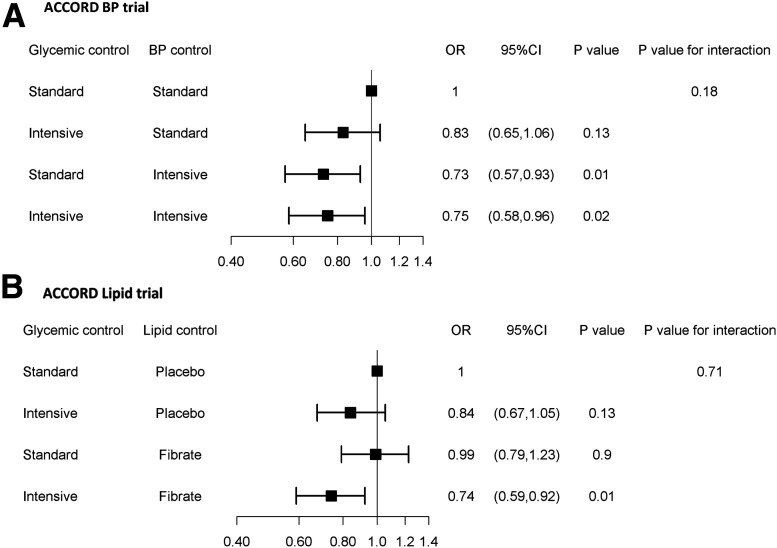
Effect of Interaction Between ACCORD Interventions on CAN
Figure 4 shows the combined effect of glycemia and the other two interventions tested in the ACCORD subtrials. The intensive glycemic intervention applied on top of intensive BP control did not appear to lower the risk of CAN to a greater extent than that achieved by intensive BP control applied alone (OR 0.75, 95% CI 0.58–0.96, vs. OR 0.73, 95% CI 0.57–0.93) (Fig. 4). However, there was no significant evidence of deviation from additivity of the two interventions (P = 0.18). In the lipid subtrial, intensive glycemic intervention had similar effects on CAN in the fenofibrate and placebo arms (Fig. 4).
Figure 4.
Effects of the combination of intensive glycemic control with intensive BP control (ACCORD BP) (A) and fenofibrate (ACCORD Lipid) (B) on CAN risk.
Conclusions
This study examined the effects of intensively treating hyperglycemia, hypertension, and dyslipidemia on CAN risk in a large cohort of T2D participants in the ACCORD clinical trial. During a median follow-up of 5 years, we observed significantly protective effects of intensive glycemic and BP interventions on CAN, with average risk reductions of 16% and 25%, respectively. The effect of the intensive glycemic intervention on incident CAN persisted after transition to the standard glycemic control targets, which suggests a “metabolic memory” phenomenon such as that demonstrated in prior cohorts and for other diabetes complications (7,18). Our data also suggest differences in the effectiveness of intensive glycemic control based on the presence or absence of CVD history and in the effectiveness of BP control based on age. If confirmed by further studies, these findings may provide a rationale for prioritizing interventions based on patients’ characteristics.
Our findings are consistent with those of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC), which demonstrated a strong protective effect of intensive glycemic control on CAN (OR 0.69, 95% CI 0.51–0.93) among patients with type 1 diabetes (19) that continues over time (20), but are at variance with those of the Veterans Affairs Diabetes Trial (VADT), in which intensive glycemic control did not show any beneficial effect on autonomic neuropathy among T2D subjects. However, in VADT, autonomic dysfunction was defined as a composite of self-reported symptoms of orthostatic hypotension, gastroparesis, neurologic bladder, and diabetic diarrhea, and no objective measures of CAN were available (21). Self-reported symptoms have low sensitivity and specificity, and orthostatic hypotension is unspecific as an index of CAN, especially in a population with multiple comorbidities requiring multiple medications, many of which could have promoted orthostatic hypotension. This, combined with the heterogeneous nature of the composite outcome, may have hampered detection of the effect of intensive glycemic control.
Multiple studies have established hypertension as a risk factor for CAN in T2D (1). However, data on whether improved BP control has beneficial effects on CAN are scant. The Atherosclerosis Risk in Communities (ARIC) study showed that after 9 years of follow-up of individuals with hypertension, those who were treated with antihypertensives had significantly higher SDNN and rMSSD than those who were not treated (22). A previous ACCORD study showed that intensive BP control did not predispose patients to orthostatic hypotension (23), which may have been partly due to the protective effects of intensive BP control on CAN. These findings were consistent with data showing that long-term BP control can reset baroreflex sensitivity (24), thereby preventing autonomic function from worsening in T2D.
High serum triglycerides are also independent predictors of CAN in T2D (25,26), and increasing mechanistic evidence has implicated fenofibrate—an agonist of peroxisome proliferator–activated receptor-α (PPAR-α) with triglyceride-lowering properties—as having beneficial effects on autonomic function. For instance, this drug was shown to increase baroreflex sensitivity via UCP2 upregulation and/or through increased neuronal vasoconstrictor response in carotid arteries (27,28). Also, fenofibrate-mediated reduction of certain lipids, such as sphingolipids, appears to be beneficial for the autonomic system (29,30). Despite this prior evidence, however, treatment with fenofibrate was not significantly associated with prevention of CAN in ACCORD. Consistent with the results of a randomized, open-label trial of individuals with combined hyperlipidemia (31), we observed some salutary effects of fenofibrate on SDNN and rMSSD considered individually as continuous variables, but these were not sufficient to lower the risk of CAN as defined by both of these indices being below the fifth percentile of their general population distributions.
Current American Diabetes Association guidelines recommend a multifactorial approach to the prevention of CAN in T2D, largely based on evidence from the Steno-2 trial (1). Steno-2 interventions targeted BP (SBP <140 mmHg and DBP <85 mmHg in 1993–1999 and SBP <130 mmHg and DBP <80 mmHg in 2000–2001), HbA1c (<6.5%), triglycerides (<150 mg/dL), and total cholesterol (<190 mg/dL in 1993–1999 and <175 mg/dL in 2000–2001). Such a multipronged approach led to a large reduction of autonomic neuropathy risk (relative risk 0.37, 95% CI 0.18–0.79, P = 0.002) (10), presumably resulting from the summed effects of the different interventions applied in this study. Since the Steno-2 targets approximately correspond to those of the standard treatments in ACCORD, our findings suggest that a more intensive application of these interventions may further improve their effectiveness in preventing CAN. As CAN presentation varies, from subclinical cardiovascular autonomic nerve dysfunction, presenting with asymptomatic changes in HRV that may be reversible, to more advanced (1,9,26), it is important and clinically relevant to document presence of CAN as early as possible to decide when and how to implement optimal strategies for risk factors management in a personalized approach.
Strengths of our study include the large sample size, the randomized design, a rich array of clinical data, and the rigorous, long-term follow-up. However, some limitations should be acknowledged. First, this was a post hoc analysis of a study that was not specifically designed for CAN, in terms of power and inclusion/exclusion criteria. Second, CAN status was based on HRV indices obtained from short electrocardiography recordings, with use of cutoffs derived from their distribution in individuals free of CVD risk factors, rather than on dynamic cardiovascular autonomic reflex tests (CARTS) such as heart rate response to deep breathing, standing, and Valsalva maneuver (1). However, while the definition of CAN used in our study may have been suboptimal, the very large sample size of valid evaluations available likely offset the loss of power that may have resulted from this. In addition, a composite of the SDNN and rMSSD variables was found to be a strong predictor of cardiovascular mortality in both type 1 diabetes and T2D (4,32) as well as in the general population (17), to the point that current guidelines recommend its use to evaluate CAN in large trials (1). Third, the association between treatments and CAN may have been biased by the presence of clinical comorbidities and drug treatments (22) that can affect HRV. However, adjustment of the analysis by time-dependent indicators of these drugs and cardiovascular events did not alter the effects of the intensive glycemic intervention. While such beneficial effects of treatments on CAN were robust, the translation of these benefits on important clinical sequelae of CAN, such as mortality and severe hypoglycemia, could not be assessed, since intensive glycemic therapy was associated, for reasons unrelated to CAN, with higher rates of those events in ACCORD. Fourth, since ACCORD was completed several years ago, we cannot extrapolate the effects on CAN observed in the current study to those that one may obtain with newer glucose-lowering drugs such as glucagon-like peptide 1 receptor agonists and sodium–glucose cotransporter 2 inhibitors. Finally, ACCORD was specifically designed for patients at high cardiovascular risk. Whether these conclusions can be generalized to other patients with T2D remains to be seen.
Conclusion
In summary, this study demonstrated beneficial effects of intensive glycemic and BP control on CAN occurrence. Such benefits should be carefully weighed against the risks and costs associated with these interventions, including the excess mortality observed with intensive glycemic control in ACCORD. The finding of possible heterogeneity in the effectiveness of intensive glycemic control based on CVD history, and of BP control based on age, may allow personalization of this treatment to maximize its cost-effectiveness.
Article Information
Acknowledgments. The authors thank the investigators, staff, and participants of ACCORD. The authors also thank Dr. Douglas Hayden for advice on data analysis as part of the Biostatistical Consulting Service of Harvard Catalyst of the Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health (NIH), award UL 1TR002541).
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the NIH.
Funding. Y.T. was supported by the Iacocca Foundation through a Mary K. Iacocca Fellowship. R.P.B. was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK/NIH) grants R01 DK107956-01 and U01 DK119083 and the JDRF Center of Excellence at the University of Michigan. The study was also supported by NIDDK/NIH grant DK36836 (Enrichment Core of the Diabetes Research Center at the Joslin Diabetes Center). The ACCORD clinical trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010); by other components of the NIH, including the NIDDK, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceuticals, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, OMRON Healthcare, Sanofi, and Schering-Plough. H.C.G. reports grants from NHLBI during the conduct of the study. A.D. reports grants from NHLBI, NIDDK, and JDRF, outside the submitted work. R.P.B. reports grants from NHLBI, grants from NIDDK, and grants from NIDDK, during the conduct of the study.
The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding entities.
Duality of Interest. J.M. reports personal fees from Novo Nordisk, grants from the National Dairy Council, personal fees from dairy farmers, and grants from Kowa Research Institute, outside the submitted work. H.C.G. reports grants from Sanofi, personal fees from Sanofi, grants from Eli Lilly, personal fees from Eli Lilly, grants from AstraZeneca, personal fees from AstraZeneca, grants from Boehringer Ingelheim, personal fees from Boehringer Ingelheim, personal fees from Abbott, grants from Novo Nordisk, personal fees from Novo Nordisk, grants from Merck, personal fees from Merck, personal fees from Jannsen, grants from Abbott, and personal fees from Kowa Research Institute, outside the submitted work. V.F. reports grants (to Tulane University) from Asahi and Bayer; honoraria for consulting and lectures from Takeda, Novo Nordisk, Sanofi, and AstraZeneca; and stock options from Microbiome Technologies, BRAVO4Health, and Insulin Algorithm. A.D. reports funding from Sanofi outside the submitted work. R.P.B. reports grants from AstraZeneca, personal fees from Bayer, personal fees from Boehringer Ingelheim, and personal fees from Novo Nordisk, outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.T. and A.D. acquired and analyzed the data. Y.T., A.D., and R.P.B. designed the study, interpreted the data from ACCORD, wrote the initial draft of the manuscript, and revised the manuscript to its final form. H.S., C.R.B.J., X.S., J.M., M.S., L.S., H.C.G., and V.F. contributed to interpreting the data and revised the manuscript to its final form. R.P.B. and A.D. contributed equally to this work. A.D. and R.P.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
Clinical trial reg. no. NCT00000620, clinicaltrials.gov
A.D. and R.P.B. contributed equally to this work and are co–senior authors.
This article contains supplementary material online at https://doi.org/10.2337/figshare.13020338.
References
- 1.Pop-Busui R, Boulton AJ, Feldman EL, et al. . Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME; Diabetes Prevention Program Research Group . The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care 2006;29:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler D, Voss A, Rathmann W, et al.; KORA Study Group . Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia 2015;58:1118–1128 [DOI] [PubMed] [Google Scholar]
- 5.Laitinen T, Lindström J, Eriksson J, et al. . Cardiovascular autonomic dysfunction is associated with central obesity in persons with impaired glucose tolerance. Diabet Med 2011;28:699–704 [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pop-Busui R, Braffett BH, Zinman B, et al.; DCCT/EDIC Research Group . Cardiovascular autonomic neuropathy and cardiovascular outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2017;40:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–381 [PubMed] [Google Scholar]
- 9.Spallone V Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J 2019;43:3–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 11.Griffin SJ, Rutten GEHM, Khunti K, et al. . Long-term effects of intensive multifactorial therapy in individuals with screen-detected type 2 diabetes in primary care: 10-year follow-up of the ADDITION-Europe cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:925–937 [DOI] [PubMed] [Google Scholar]
- 12.Silverman MG, Ference BA, Im K, et al. . Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297 [DOI] [PubMed] [Google Scholar]
- 13.Xie X, Atkins E, Lv J, et al. . Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387:435–443 [DOI] [PubMed] [Google Scholar]
- 14.Accord Study Group; Buse JB, Bigger JT, Byington RP, et al. . Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99:21i–33i [DOI] [PubMed] [Google Scholar]
- 15.Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pop-Busui R, Evans GW, Gerstein HC, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neal WT, Chen LY, Nazarian S, Soliman EZ. Reference ranges for short-term heart rate variability measures in individuals free of cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). J Electrocardiol 2016;49:686–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 19.Pop-Busui R, Herman WH, Feldman EL, et al.; DCCT/EDIC Research Group . DCCT and EDIC studies in type 1 diabetes: lessons for diabetic neuropathy regarding metabolic memory and natural history. Curr Diab Rep 2010;10:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braffett BH, Gubitosi-Klug RA, Albers JW, et al.; DCCT/EDIC Research Group . Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2020;69:1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 22.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension 2003;42:1106–1111 [DOI] [PubMed] [Google Scholar]
- 23.Fleg JL, Evans GW, Margolis KL, et al. . Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension 2016;68:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salman IM Major autonomic neuroregulatory pathways underlying short- and long-term control of cardiovascular function. Curr Hypertens Rep 2016;18:18. [DOI] [PubMed] [Google Scholar]
- 25.Jaiswal M, Divers J, Urbina EM, et al.; SEARCH for Diabetes in Youth Study Group . Cardiovascular autonomic neuropathy in adolescents and young adults with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Cohort Study. Pediatr Diabetes 2018;19:680–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen ST, Witte DR, Fleischer J, et al. . Risk factors for the presence and progression of cardiovascular autonomic neuropathy in type 2 diabetes: ADDITION-Denmark. Diabetes Care 2018;41:2586–2594 [DOI] [PubMed] [Google Scholar]
- 27.Guan J, Zhao M, He C, et al. . Anti-hypertensive action of fenofibrate via UCP2 upregulation mediated by PPAR activation in baroreflex afferent pathway. Neurosci Bull 2019;35:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Campo L, Blanco-Rivero J, Balfagon G. Fenofibrate increases neuronal vasoconstrictor response in mesenteric arteries from diabetic rats: role of noradrenaline, neuronal nitric oxide and calcitonin gene-related peptide. Eur J Pharmacol 2011;666:142–149 [DOI] [PubMed] [Google Scholar]
- 29.Othman A, Benghozi R, Alecu I, et al. . Fenofibrate lowers atypical sphingolipids in plasma of dyslipidemic patients: a novel approach for treating diabetic neuropathy? J Clin Lipidol 2015;9:568–575 [DOI] [PubMed] [Google Scholar]
- 30.Nestel PJ, Khan AA, Straznicky NE, et al. . Markers of sympathetic nervous system activity associate with complex plasma lipids in metabolic syndrome subjects. Atherosclerosis 2017;256:21–28 [DOI] [PubMed] [Google Scholar]
- 31.Melenovsky V, Wichterle D, Simek J, et al. . Effect of atorvastatin and fenofibrate on autonomic tone in subjects with combined hyperlipidemia. Am J Cardiol 2003;92:337–341 [DOI] [PubMed] [Google Scholar]
- 32.Ziegler D, Zentai CP, Perz S, et al.; KORA Study Group . Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care 2008;31:556–561 [DOI] [PubMed] [Google Scholar]