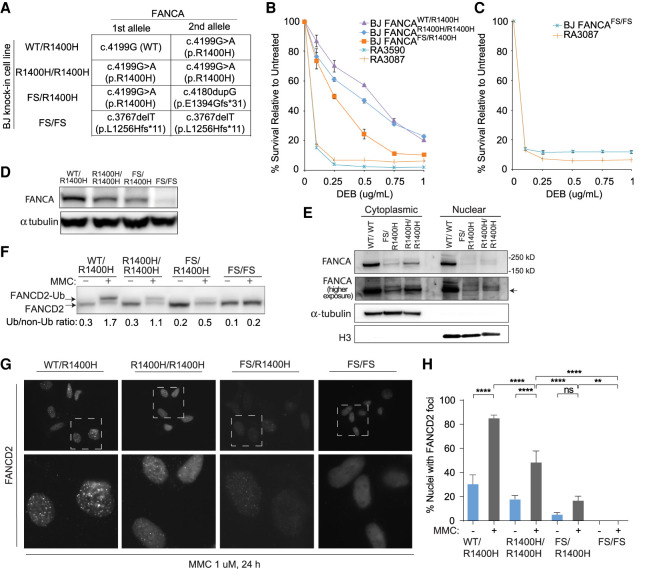
Figure 3.
Genome editing of wild-type fibroblasts to express p.R1400H FANCA endogenously results in decreased FANCA levels and impaired FANCD2 ubiquitination and foci formation upon MMC-induced damage. (A) Genotypes and predicted effects of CRISPR-mediated FANCA variant knock-in clones including heterozygous (WT/R1400H), homozygous (R1400H/R1400H), compound heterozygous frameshift (FS/R1400H), and homozygous frameshift (FS/FS) were generated in BJ fibroblasts. (B,C) DEB cellular sensitivity assays showing survival of patient cell lines in comparison to FANCA variant knock-in clones. (D) Western blot assessing endogenous FANCA expression in untreated BJ fibroblast FANCA clones. (E) Western blot assessing endogenous FANCA upon cellular fractionation of untreated FANCA variant knock-in clones. (F) Western blot assessing FANCD2 monoubiquitination in BJ fibroblast FANCA clones. Cells were either untreated or cultured with 1 µM MMC for 24 h. Relative ratio of monoubiquitinated to nonubiquitinated FANCD2 was measured for each variant. (G) Images of immunofluorescence of FANCD2 foci in BJ fibroblast FANCA clones. Lower panel images are magnified to demonstrate differences in foci formation. Cells were preextracted prior to fixation. (H) Quantification of cells observed to have FANCD2 foci in BJ fibroblast FANCA clones by immunofluorescence. Cells were either untreated or cultured with 1 µM MMC for 24 h. Statistical significance determined by ordinary one-way ANOVA with multiple comparisons. (****) P < 0.0001; (**) 0.0021 < P < 0.0002.