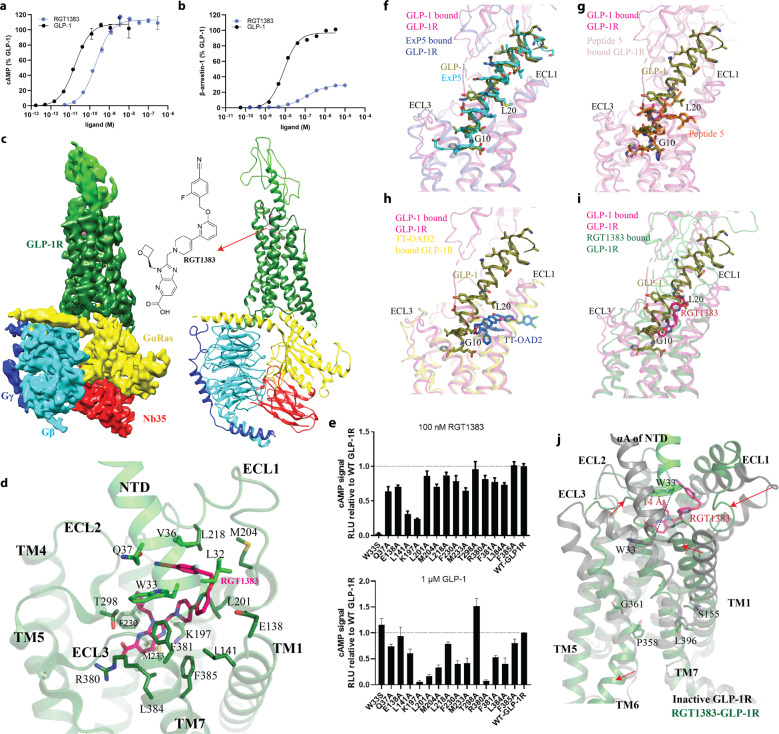
Fig. 1. Structure of RGT1383–GLP-1R–Gs complex.
a, b Comparison of the effects between RGT1383 and GLP-1 on cAMP accumulation and β-arrestin recruitment. c Cryo-EM map and structure model of the complex. d The binding mode of RGT1383 in binding pocket of GLP-1R. e The mutagenesis analysis of residues in RGT1383-binding pocket of GLP-1R. Upper, 100 nM RGT1383; lower, 1 μM GLP-1. f–i Agonists occupy different positions in the orthosteric binding pocket. GLP-1 (deep olive), ExP5 (cyan), Peptide 5 (orange), TT-OAD2 (deep blue) and RGT1383 (hot pink) bound active GLP-1R structures are shown in light magenta, light blue, pink, yellow and deep green, respectively. j Structural comparison of RGT1383-bound GLP-1R with inactive GLP-1R (PDB code: 7C2E and 6LN2). Arrows show the extent of motion.