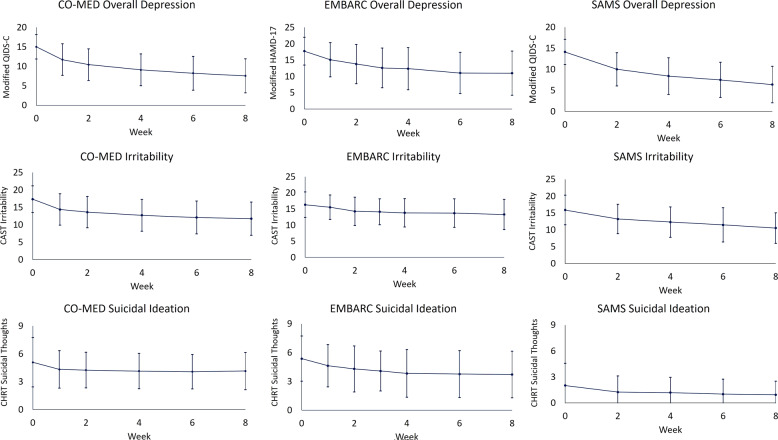
Fig. 1. Levels of overall depression, irritability, and suicidal ideation, during CO-MED, EMBARC, and SAMS trials.
CO-MED is Combining Medications to Enhance Depression Outcomes (n = 665), EMBARC is Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care (n = 296), and SAMS is Suicide Assessment Methodology Study (n = 266). Irritability was measured with the five-item irritability domain of Concise Associated Symptom Tracking (CAST) scale. Suicidal ideation was measured with three-item suicidal thoughts factor of Concise Health Risk Tracking (CHRT) scale. After removing the suicide-related item, overall depression was assessed with the Hamilton Depression Rating Scale (modified HAMD-17) in the EMBARC trial, and with Quick Inventory of Depressive Symptomatology Clinician-Rated version (modified QIDS-C) in the CO-MED and SAMS trials. Error bars represent 1 standard deviation above and below observed means at each visit.