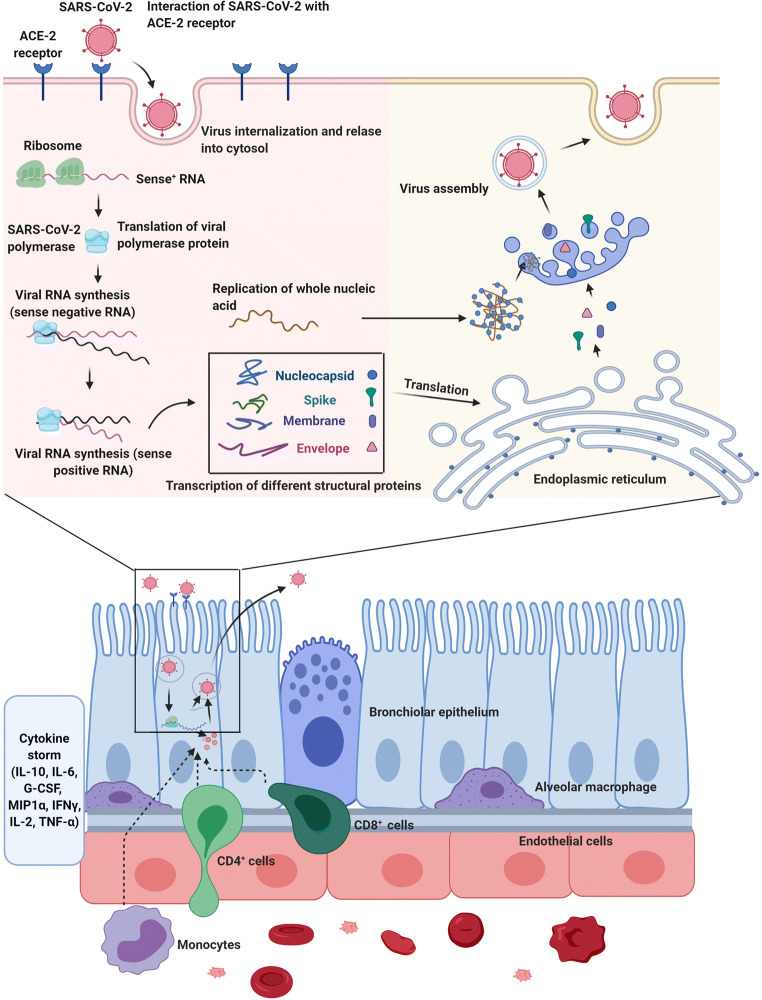
Fig. 2.
The proliferation of SARS-CoV-2 within the host cells initiates soon after attachment of S protein (S1 and S2) to the cell membrane-bound ACE-2 receptor. Allosteric changes in S protein promote viral envelope fusion with the cell membrane through endosomal signaling. Inside the cells, the genomic pool is released, transcribed, and translated to synthesize various components of viral structure. Finally, viral proteins and genome RNA are assembled into virions in the endoplasmic reticulum and Golgi apparatuses and transported into microvacuoles. In the next, step, the microvesicles containing virions are released. After infection of host cells with COVID-19 nanoparticles, the release of virions promotes pyroptosis and massive cellular damage. The neighboring cells such as endothelial cells, dust cells (alveolar macrophages) start to release an array of cytokines and chemokines. With the progression of cellular damage, blood lymphocytes (either T and B), as well as macrophages, are recruited to the site of infection. Accumulation of immune cells exacerbates the inflammatory responses by the continuous production of inflammatory cytokines. Interleukine 10: IL-10; Interleukine 6: IL-6; granulocyte colony-stimulating factor: G-CSF; Macrophage inflammatory protein 1α: MIP1α; Interferon-gamma: IFNγ; Interleukine 2: IL-2; Tumour necrosis factor-α: TNF-α. The illustration was created with BioRender.com