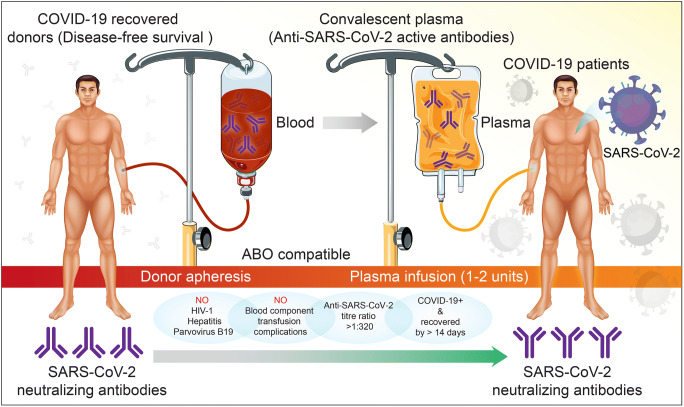
Fig. 4.
Convalescent plasma therapy. The use of convalescent plasma to treat COVID-19 requires donor testing of a person who has recovered from disease and has substantial titers of SARS-CoV-2 neutralizing antibodies. The plasma of recovered COVID-19 patients contains SARS-CoV-2 antibodies. These are present in plasma, which are collected then used as therapies. Convalescent plasma is safe, specific and effective. This schematic describes how convalescent plasma therapy is administered. It is as follows: (1) Donor apheresis. Blood is collected from the patient and antibody containing plasma is harvested by apheresis. (2) Plasma infusion. Convalescent plasma is collected from plasma of the donor then administered to the COVID-19 patient intravenously to deliver virus-specific antibodies. The plasma is collected through blood banks and given to blood-type-compatible patients with active SARS-CoV-2 infection. All donors are screened for HIV-1 and for hepatitis viruses and parvovirus B19. There are no other blood components uncovered that could yield secondary complications. The anti-SARS-CoV-2 titer must be at or greater than 1:320. Donors should have no systemic illness for at least 14 days after recovery